Abstract
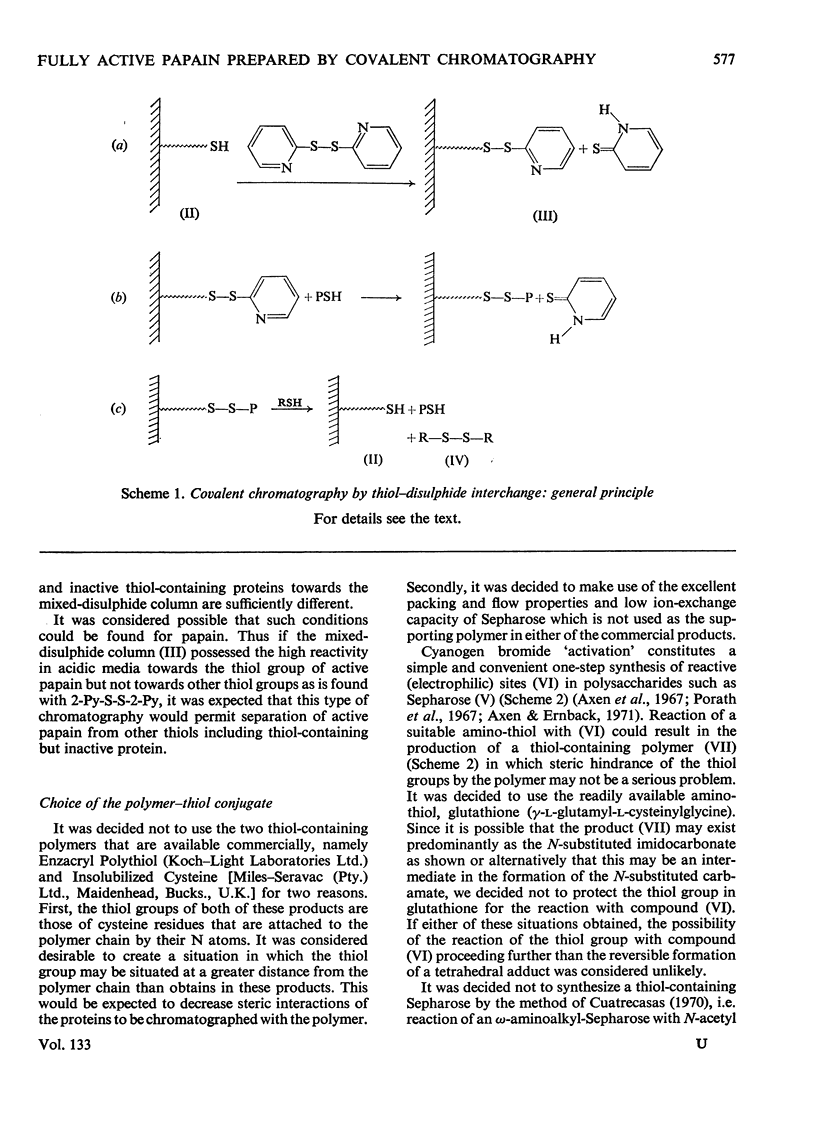
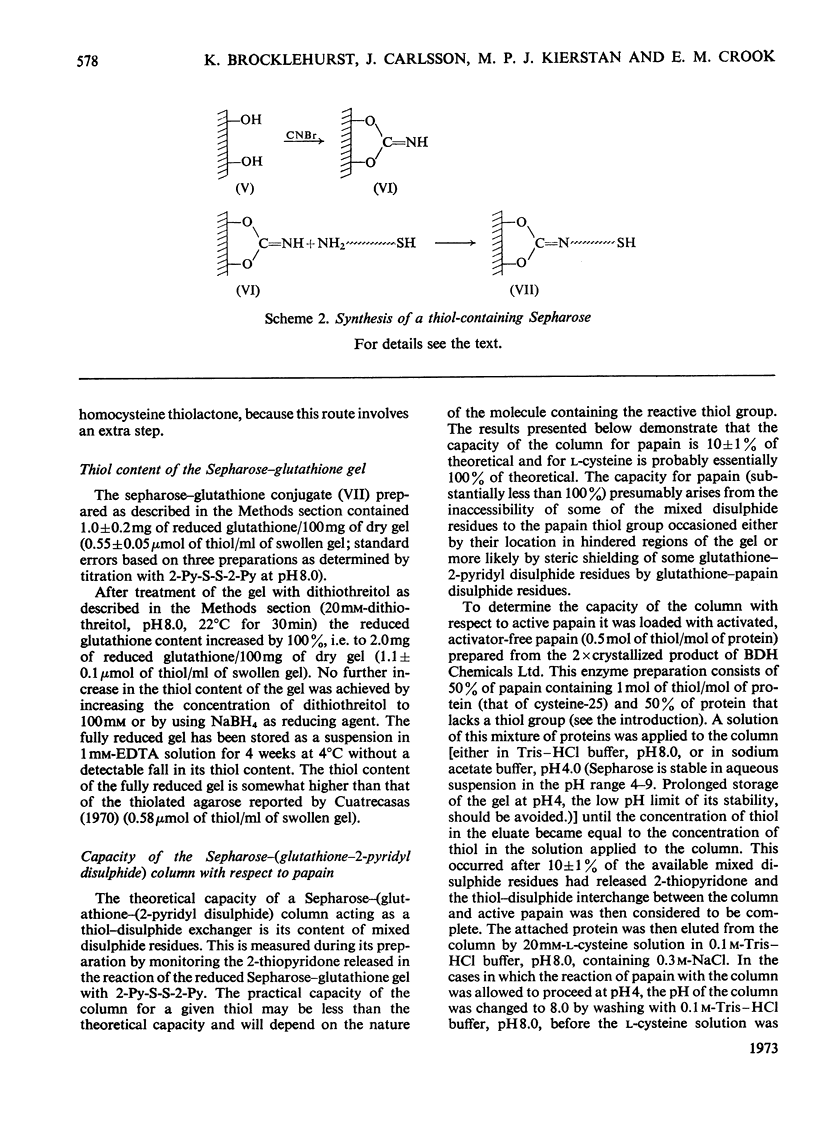
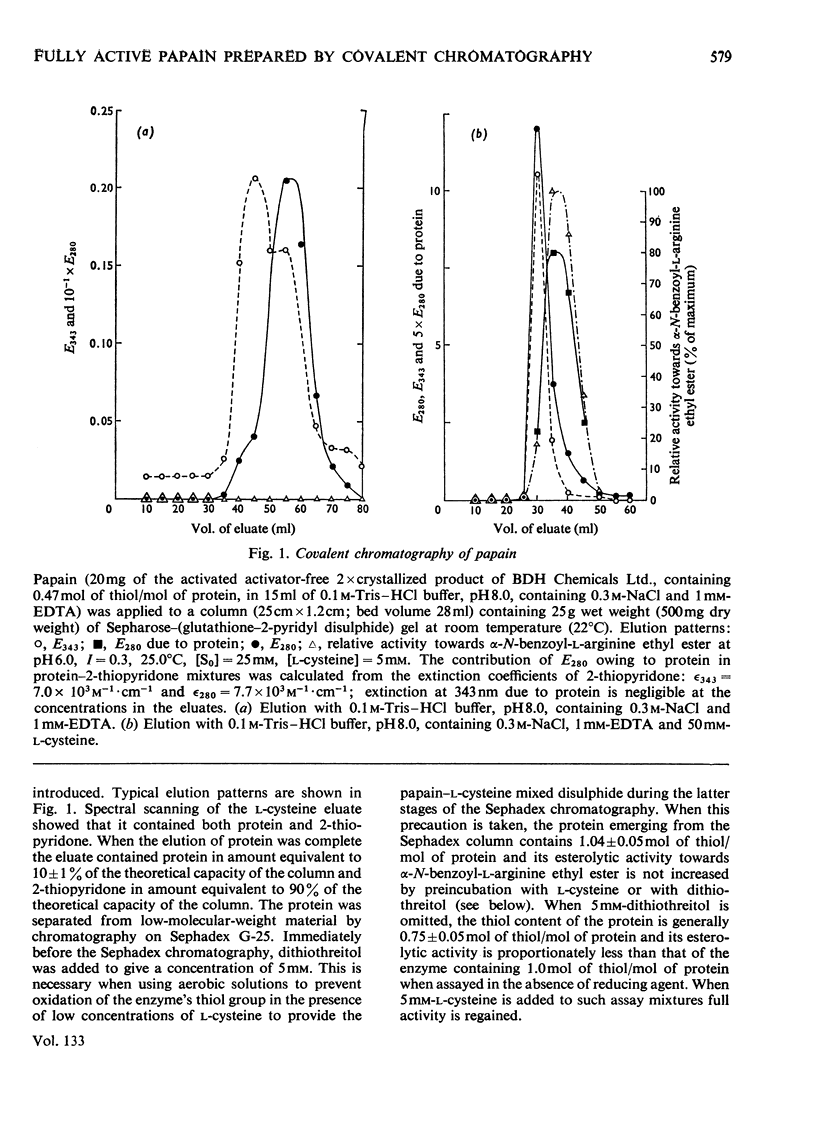
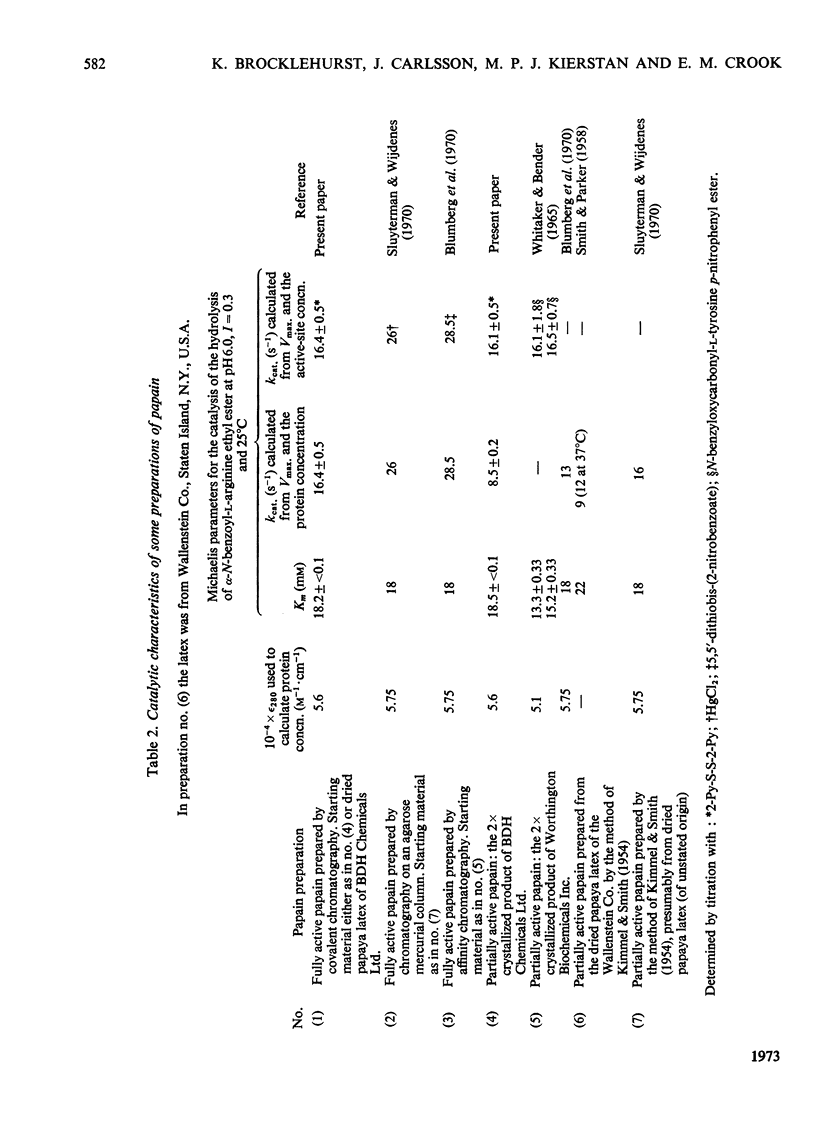
1. A Sepharose–(glutathione–2-pyridyl disulphide) conjugate has been prepared. 2. Its use in a new type of chromatography, covalent chromatography by thiol–disulphide interchange, is described. 3. With this technique, papain containing 1 intact catalytic site [thiol with high reactivity towards 2,2′-dipyridyl disulphide (2-Py-S-S-2-Py) at pH4] per mol of protein is readily prepared both from dried papaya latex and from commercial 2×crystallized partially active papain. 4. The catalysis of the hydrolysis of α-N-benzoyl-l-arginine ethyl ester at pH6.0, 25.0°C, I=0.3 by fully active papain thus prepared is characterized by Km=18.2±<0.1mm and kcat.=16.4±0.5s−1.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashani Y., Wilson I. B. A covalent affinity column for the purification of acetylcholinesterase. Biochim Biophys Acta. 1972 Jul 13;276(1):317–322. doi: 10.1016/0005-2744(72)90034-4. [DOI] [PubMed] [Google Scholar]
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bender M. L., Begué-Cantón M. L., Blakeley R. L., Brubacher L. J., Feder J., Gunter C. R., Kézdy F. J., Killheffer J. V., Jr, Marshall T. H., Miller C. G. The determination of the concentration of hydrolytic enzyme solutions: alpha-chymotrypsin, trypsin, papain, elastase, subtilisin, and acetylcholinesterase. J Am Chem Soc. 1966 Dec 20;88(24):5890–5913. doi: 10.1021/ja00976a034. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Crook E. M., Kierstan M. The mutability of stem bromelain: evidence for perturbation by structural transitions of the parameters that characterize the reaction of the essential thiol group of bromelain with 2,2'-dipyridyl disulphide. Biochem J. 1972 Jul;128(4):979–982. doi: 10.1042/bj1280979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kierstan M., Little G. The reaction of papain with Ellman's reagent (5,5'-dithiobis- (2-nitrobenzoate) dianion). Biochem J. 1972 Jul;128(4):811–816. doi: 10.1042/bj1280811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. THE SULFUR DISTRIBUTION OF PAPAIN. J Biol Chem. 1965 Jan;240:201–208. [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Klein I. B., Kirsch J. F. The mechanism of the activation of papain. Biochem Biophys Res Commun. 1969 Mar 10;34(5):575–581. doi: 10.1016/0006-291x(69)90776-1. [DOI] [PubMed] [Google Scholar]
- Little G., Brocklehurst K. Kinetics of the reversible reaction of papain with 5,5'-dithiobis-(2-nitrobenzoate) dianion: evidence for nucleophilic reactivity in the un-ionized thiol group of cysteine-25 and for general acid catalysis by histidine-159 of the reaction of the 5-mercapto-2-nitrobenzoate dianion with the papain-5-mercapto-2-nitrobenzoate mixed disulphide. Biochem J. 1972 Jun;128(2):475–477. doi: 10.1042/bj1280475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- SMITH E. L., PARKER M. J. Kinetics of papain action. III. Hydrolysis of benzoyl-L-arginine ethyl ester. J Biol Chem. 1958 Dec;233(6):1387–1391. [PubMed] [Google Scholar]
- Sluyterman L. A. The activation reaction of papain. Biochim Biophys Acta. 1967 Jul 11;139(2):430–438. doi: 10.1016/0005-2744(67)90046-0. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. The rate-limiting reaction in papain action as derived from the reaction of the enzyme with chloroacetic acid. Biochim Biophys Acta. 1968 Jan 8;151(1):178–187. doi: 10.1016/0005-2744(68)90172-1. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Staining of protein zones after isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Aug 27;243(2):345–348. doi: 10.1016/0005-2795(71)90094-8. [DOI] [PubMed] [Google Scholar]
- WHITAKER J. R., BENDER M. L. KINETICS OF PAPAIN-CATALYZED HYDROLYSIS OF ALPHA-N-BENZOYL-L-ARGININE ETHYL ESTER AND ALPHA-N-BENZOYL-L-ARGININAMIDE. J Am Chem Soc. 1965 Jun 20;87:2728–2737. doi: 10.1021/ja01090a034. [DOI] [PubMed] [Google Scholar]


