Abstract
Background
Human resistance to re-infection with S. mansoni is correlated with high levels of anti-soluble adult worm antigens (SWAP) IgE. Although it has been shown that IL-4 and IL-5 are crucial in establishing IgE responses in vitro, the active in vivo production of these cytokines by T cells, and the degree of polarization of Th2 vs. Th0 in human schistosomiasis is not known. To address this question, we determined the frequency of IL-4 and IFN-γ or IL-5 and IL-2 producing lymphocytes from schistosomiasis patients with high or low levels of IgE anti-SWAP.
Results
Our analysis showed that high and low IgE-producers responded equally to schistosomiasis antigens as determined by proliferation. Moreover, patients from both groups displayed similar percentages of circulating lymphocytes. However, high IgE-producers had an increased percentage of activated CD4+ T cells as compared to the low IgE-producers. Moreover, intracellular cytokine analysis, after short-term stimulation with anti-CD3/CD28 mAbs, showed that IgE high-producers display an increase in the percentage of T lymphocytes expressing IL-4 and IL-5 as compared to IgE low-responders. A coordinate control of the frequency of IL-4 and IL-5 producing lymphocytes in IgE high, but not IgE low-responders, was observed.
Conclusions
High IgE phenotype human schistosomiasis patients exhibit a coordinate regulation of IL-4 and IL-5 producing cells and the lymphocyte derived IL-4 comes from true polarized Th2 like cells, in the absence of measurable Th0 cells as measured by co-production of IL-4 and IFN-γ.
Background
Schistosomiasis is a disease caused by a helminth parasite and effects more than 200 million people. Infected individuals mount a strong proliferative response in vitro to parasite antigens, and anti-worm and egg antibodies are abundant as well [1,2]. Although effective treatment is available, due to socio-economical conditions of the endemic countries, re-infection is highly prevalent and may lead to the establishment of pathology.
Correlations have been made between intensity of infection and anti-parasite IgE antibody responses in human schistosomiasis [3]. It has been shown that patients with high levels of circulating IgE anti-SWAP are resistant to re-infection [4-7]. Further studies demonstrated that this immunoglobulin isotype, correlated with resistance to re-infection, was mainly directed to a specific component of SWAP, the sm22 antigen [8]. Moreover, individuals who live in an endemic area, but were never infected with S. mansoni, display high levels of anti-parasite IgE [9]. Early studies on IL-4 function have demonstrated its ability in eliciting IgE class switching [10]. Thus, it is possible that a Th2-like regulation may be involved in the development of resistance to re-infection in schistosomiasis, through the induction of high levels of protective IgE. Moreover, IL-5, a Th2-derived cytokine, is critical in the generation of eosinophils, which are involved in mechanisms that lead to protection in human and experimental schistosomiasis [11,12].
Many mouse model systems have been used to study the interaction of the immune system with S. mansoni, and the data are mixed as to the role of Th1 and Th2 type responses in the development of protection, progression of pathology, and establishment of granulomas [13,14]. Furthermore, the role that IgE may play in experimental infections is, with respect to the role of Th1/Th2 populations, controversial. While it has been shown that IgE deficient mice or anti-IgE treated mice have higher worm burden, granuloma formation is modulated in these mice [15]. Furthermore, recent studies have demonstrated that blocking of IL-13, a Th2 derived cytokine, is accompanied by a decrease in the levels of total IgE and modulation of pulmonary granuloma formation [16].
Correlation between high IL-4 levels and elevated IgE production in conditions such as allergic reactions is well established in the literature [17]. However, most studies demonstrating the involvement of IL-4 in IgE responses have been performed in vitro, demonstrating the ability of the cells to produce IL-4 upon stimulation in culture. Whether these cells are actively controlling the immune profile in vivo, and are actively required to maintain the IgE production remains to be directly addressed. In the present study we performed an ex vivo analysis of cytokine production by lymphocytes isolated from schistosomiasis patients that produced high or low levels of IgE anti-SWAP. To determine the nature of the active immunoregulation taking place in these infected patients, we performed an analysis of the cytokine expression pattern and frequency of cytokine expressing lymphocytes for key Th1 and Th2 associated cytokines. These cytokine expression patterns and frequencies were then compared to the immuno-phenotype of high or low anti-SWAP IgE.
Results
PBMC from IgE-high and low producers mounted equivalent proliferative responses to S. mansoni antigens
PBMC from both groups were simulated for five days and proliferation measured by 3H-Thymidine uptake. As shown in figure 1, the responses of the two groups was intense to both SEA and SWAP, with no statistically significant differences between the two (p = 0.1). The response of the two groups to the positive control, SEB, demonstrated the potential of each individual to mount an equally strong T cell response independent of their group definition and the nature of schistosoma-related antigen.
Figure 1.
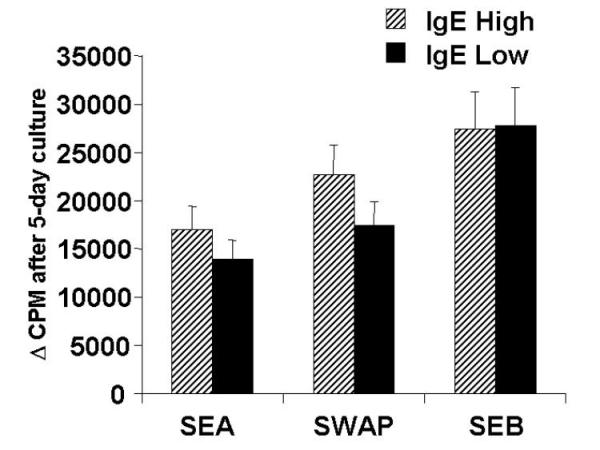
Proliferative response of PBMC from IgE high and low responder groups. PBMC were stimulated in vitro with SEA (25 ug/ml), SWAP (25 ug/ml), and the superantigen positive control, SEB (0.1 ug/ml). The bars represent the mean proliferate response and standard error of the response. None of the differences were statistically significant using students T test.
IgE high responder patients expressed a higher frequency of activated CD4+ T cells than the IgE low responders
Lymphocytes from patients of both groups were compared for percentage of cells expressing CD4, CD8, CD19, and for co-expression of the activation marker HLA-DR in the CD4+ and CD8+ T cell populations. Figure 2 demonstrates that the percentage of CD4+, CD8+, or CD19+ cells were not different between the two groups. However, the percentage of activated CD4+ T cells was significantly higher in the IgE high responder group.
Figure 2.
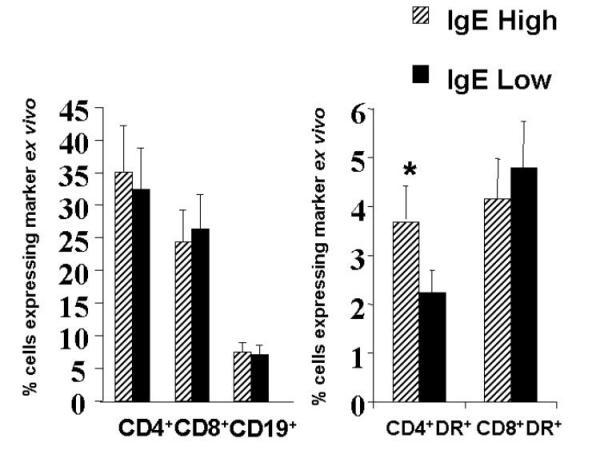
Ex vivo lymphocyte profile using flow cytometry revealed a higher percent of activated CD4+ T cells in the IgE high responder group. Lymphocytes freshly isolated from whole blood were stained for the indicated markers and the percent of cells in the lymphocyte gate expressing each of the shown markers are diagrammed as mean with the standard error. * p < 0.05 using the students T test.
IgE high responder individuals express a higher frequency of IL-4 and IL-5 producing lymphocytes, the former coming from exclusively Th2 like cells
In order to gain a better understanding of the immunoregulation and balance of cytokine production in these individuals, we performed single cell cytoplasmic staining using two pairs of labeled antibodies as described in Materials and Methods. Figure 3 shows a representative dot-plot of cytokine production by lymphocytes of high (A-C) and low (D-F) -anti-SWAP IgE responders, demonstrating the clearly defined cytokine-producing cell populations. Moreover, it can be seen that greater than 90% of the IL-4 producing cells are polarized Th2 like cells, with little contribution from Th0 cells as indicated by the lack of double positive lymphocytes producing both IL-4 and IFN-γ (Figure 3B), confirming the polarization of Th2 type cells in these individuals.
Figure 3.
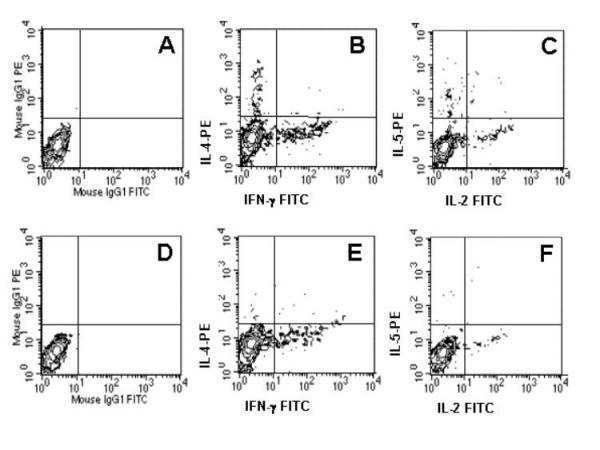
Th1 and Th2 cytokines are made by separate, Th1 and Th2 like cells, not by double producing, Th0 cells. Representative cytokine production (IL-4 and IFN-γ or IL-5 and IL-2) of total gated lymphocytes from high (A-C) or low IgE-producers (D-F). Isotype controls show the lack of non-specific staining (A and D). The absence of double producing, IL-4+/IFN-γ+, lymphocytes is evident in the high IgE group (B), with essentially all IL-4 producing cells being negative for IFN-γ production. In all cases, there is a clear segregation of IL-4 and IFN-γ producing cells (B and E), or of IL-5 and IL-2 producing cells (C and F).
As seen in figure 4, the IgE high responder group had a significantly higher frequency of cells producing IL-4 (1.11%) and IL-5 (0.41%) after short term stimulation with anti-CD3/CD28 mAbs than did the IgE low responder group (0.31 and 0.09%, respectively). In contrast, the IgE high and low responder groups did not differ in the percentage of cells expressing IL-2 or IFN-γ (Figure 4). Non-stimulated controls showed undetectable levels of all cytokines tested (data not shown).
Figure 4.
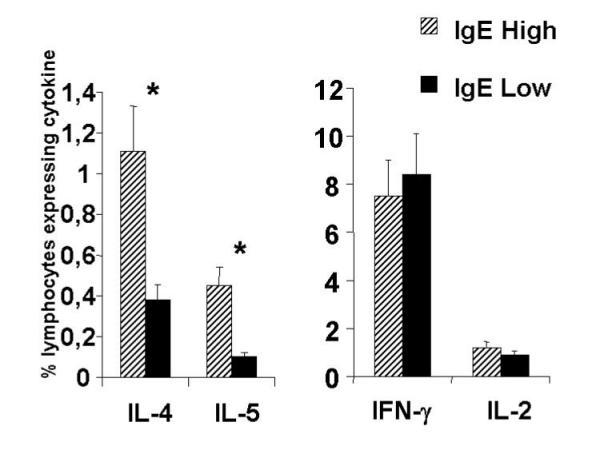
Lymphocytes from IgE high responder (n = 9) patients have a higher frequency of IL-4 and IL-5 expressing cells than the IgE low responder (n = 9) patients. Lymphocytes from the two groups of individuals were stimulated for a total of 11 h with anti-CD3, CD28, and IL-2 and stained with anti-IL-4-PE and IFN-γ-FITC or anti-IL-5-PE and IL-2-FITC as described in Materials and Methods. The data represent the mean percent of lymphocytes expressing the indicated cytokine from the two groups with their standard error. * p < 0.05 using students T test.
A further analysis was performed looking at the ratio of the percentage of cells expressing the Th2 type cytokine IL-4, critical for IgE class switching, as compared to the percent expressing the Th1 associated cytokine, IFN-γ. This ratio was three times higher in the IgE high responding group than the IgE low responding group (data not shown).
Finally, when comparing the frequency of IL-4 and IL-5 producing cells in each individual from the high responder and low responder anti-SWAP IgE group, we observed a striking difference in the coordinate control of the frequency of cytokine producing cells. As seen in figure 5, we observed a strong coordinate control (c.c. = 0.93) of the frequency of IL-4 and IL-5 producing lymphocytes only in the high IgE anti-SWAP responders. Moreover, this relationship was not seen for the frequency of cells producing IFN-γ and IL-2 (Figure 5) for either of the groups.
Figure 5.
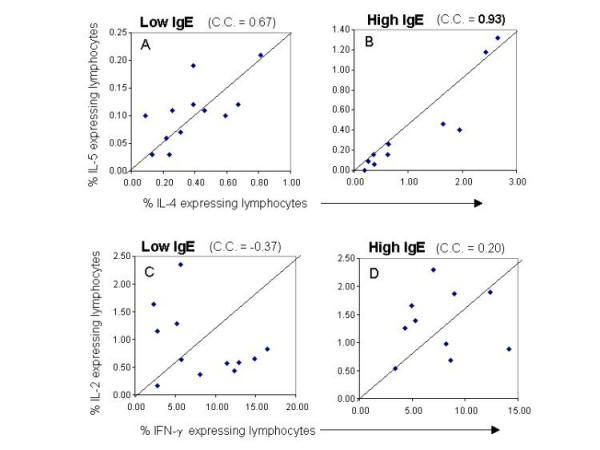
Coordinate regulation of the frequency of IL-4 and IL-5 expressing lymphocytes from high or low anti-SWAP IgE responders. The data is presented in a correlation plot considering IL-4 and IL-5 or IFN-γ and IL-2 for individual patients from the IgE low (A,C) and high (B,D) groups. The correlation coefficient was calculated using Jump Statistics program.
Discussion
A vast number of studies have been performed in human and experimental schistosomiasis in order to understand the dynamics of interactions between host and parasite and the pathological outcome of this complex relationship. Several important findings have been acquired over the past years but the exact mechanisms through which pathology or resistance to infection is established are not known. It is clear that the host's immune system plays a key role in this decision. Early studies have demonstrated that patients with different clinical forms of schistosomiasis respond in vitro to parasite antigens as well as to idiotypes [2,18-20]. Nonetheless, patients from different endemic countries, or even different areas within the same country, display distinct degrees of reactivity to a given antigen or preferentially respond to different antigenic preparations [21,22,19]. Also, cytokine production in human schistosomiasis has been accessed in different systems [23,24] and, to date, no definitive correlation between a given cytokine profile and the development of pathology or protection has been well established in human disease.
It has previously been shown that intestinal and hepatosplenic schistosomiasis patients display higher frequency of CD3+HLA-DR+ circulating cells, as compared to non-infected individuals [25]. Moreover, it has been demonstrated that activated T cells account for the production of the majority of cytokines in the mouse [26]. Also, a high frequency of activated T cell has been found in the peripheral blood of individuals chronically infected with Trypanosoma cruzi[27] and PBMC from these patients were shown to produce high levels of immuno-regulatory cytokines [28].
In our present study, patients with the high anti-SWAP IgE phenotype displayed higher levels of T cell activation as well as presented with higher frequency of IL-4 and IL-5 producing cells. Low IgE-responders displayed lower frequency of activated CD4+ T cells and a lower frequency of cells producing these cytokines. This suggests that active production of IL-4 and IL-5 by T cells is maintaining the IgE phenotype in these chronically infected individuals. Interestingly, the differences in T cell activation and cytokine production seen ex vivo in patients from each group was not reflected in the proliferative responses or in the frequency of IFN-γ or IL-2 producing lymphocytes. Moreover, both groups, independent of anti-SWAP IgE levels, had equivalent levels of anti-SWAP IgG.
IgE responses to parasite antigens have been shown to be correlated with resistance to re-infection by many groups, looking at patients from different geographical areas, as well as infection by different schistosome species [7,29-31]. Although induction of IgE responses have been correlated with IL-4 production in other diseases [17], most studies were performed in vitro, reflecting the potential of IL-4 production but not accessing directly ex vivo the production of this cytokine by lymphocytes. In this study, we analyzed ex vivo, after short term stimulation with anti-CD3/anti-CD28, the cytokine production by schistosomiasis patients that were classified based on their high or low production of anti-SWAP IgE. This approach gives important information concerning the regulation of this immunological profile previously correlated with resistance, since it reveals a "picture" of cytokine production in vivo. Moreover, considering that schistosomiasis is a chronic disease, it is critical to understand whether cytokines are important in establishing as well as in maintaining a given phenotype. In the particular case of IgE responses, it is well determined that IL-4 is a switch factor for IgE but, whether it is critical for maintaining the response for long periods of time is not necessarily a given fact.
The short term stimulation used in our analysis was designed to reveal the cytokine profile of the existing activated and/or memory cells without possible influences of longer cultures. A recent study has used a similar approach to determine cytokine profiles of individuals with allergy or autoimmune diseases using, PMA/ionomycin as source of stimulus [32]. Although PMA/ionomycin stimulation leads to a higher frequency of cytokine-producing cells (data not shown), it is less likely to only amplify and reveal the cytokine profile of lymphocytes seen in vivo than anti-CD3/anti-CD28. Given our results and those of others [33] PMA/Ionomycin can skew responses as well as stimulate non-T cell populations. Thus, stimulation with anti-CD3 plus anti-CD28 is a more physiologic stimuli than PMA/Ionomycin.
Considering that IL-4 can also be produced by other cell types, involved with innate immune responses that may likely occur in the early phases of the disease, we can not rule out the possibility that other sources of IL-4 would be important for the initial production of IgE in vivo. However, considering the crucial role of T cells in chronic infections, these results strongly suggest that the specific IgE response seen in these patients is under the control of Th2 cells. Corroborating with this hypothesis, we directly demonstrated in these studies a coordinate regulation of the frequency of IL-4 and IL-5 producing cells only in the high anti-SWAP IgE responders (Figure 5). Based on previous findings studying Th2 phenotype and development [34], as well as parallel control of IL-4 and IL-5 producing cells in human schistosomiasis [35], it is likely that the same cells are producing these cytokines. It is formally possible that they come from distinct cells of a partial Th2 like phenotype that are under the same control. However, given that the IL-4 producing cells did not produce IFN-γ and vice versa, we have shown a true Th2 type polarization in these individuals (Figure 3). Many studies in vitro have demonstrated the ability of sub-populations of T cells to effect the development of neighboring cells in culture. In addition, the elucidation of the cytokine profile and balance between key immunoregulatory cytokines in these individuals should help in approaches designed to manipulate the cytokine profile and immune response in infected individuals or in vaccine development. The question as to whether these individuals have some predisposition to develop the high IgE phenotype, and if this predisposition was in fact the expression of a higher frequency of cells producing IL-4 and IL-5, has not been resolved. Moreover, it is possible that in these individuals some factor(s) other than gender, age, intensity of infection, and number of previous treatments may have led to presentation of antigens in such a way as to generate the high anti-SWAP IgE response. Further studies looking at sub-populations of T cells and their responses over time may help to clarify these questions. In conclusion, these studies demonstrate an ongoing active production of IL-4 by Th2 like cells in the absence of Th0 cells.
Conclusions
These studies demonstrate that the increase in IL-4 production in IgE high responder schistosomiasis patients comes from true Th2 like cells and not from Th0 cells. Moreover, this active Th2 polarization takes place without a decrease (or increase) in the frequency of IFN-γ or IL-2 producing cells. Thus, a specific increase in the frequency of true Th2 like cells (not Th0) accompanies the high IgE responder profile seen in human schistosomiasis, demonstrating the likely importance of these cells in maintaining this phenotype.
Materials and Methods
Patients
The individuals analyzed in this study were volunteers, part of a large study group from the S. mansoni endemic area of Corrego do Bernardo, MG, Brazil. All patients presented with the intestinal clinical form, as determined by health exams including an overall physical and palpation for symptoms of hepatosplenic disease. Any individuals that presented with positive feces exams were treated, independent of the participation in this study. Intestinal schistosomiasis patients were analyzed for anti-SWAP IgE and IgG responses and two groups were established. One group was composed of 9 individuals presenting high titers of IgE anti-SWAP (OD greater than 0.1) and the second group had 9 individuals that produced low levels of these specific antibodies (OD lower than 0.1). This cutoff was determined as described by Webster et al. [36], based on the limits of detection and the range of values of the ELISA. The levels of IgG anti-SWAP did not vary between groups. Additionally, as described by Webster et al.[36], the subset of individuals used in this study were selected based on gender and age and were found to be statistically equivalent based on race, egg burden, number of previous treatments, and co-infection with hook-worm.
In vitro proliferative analysis
In vitro proliferative responses of peripheral blood mononuclear cells (PBMC) were performed according to the protocol described by Gazzinelli et al [18]. Briefly, PBMC obtained by separating blood cells in a Ficoll gradient were washed 3 times in media, counted and cultured in the presence or absence of different stimuli for 5 days at the concentration of 2.5 × 105cells/well. Stimuli used in the cultures included SEA, SWAP, (both at 25 ug/ml final concentration) and, as a positive control, the superantigen SEB (at 0.5 ug/ml final concentration). After the incubation period, cultures were exposed to 0.5 mCi of 3[H]-thymidine for 6 hours, harvested and the incorporated radioactivity measured in an automatic scintillation counter. All cultures were performed in triplicate. Proliferative response was calculated using the mean of triplicate cultures with antigen minus the mean of triplicate cultures with medium alone (Δcpm) for each individual patient.
Ex vivo analysis of lymphocyte profiles
2 × 105 PBMC from schistosomiasis patients were incubated with FITC or PE-labeled antibody solutions for 20 minutes at 4°C. Then, preparations were washed with 0.1% sodium-azide phosphate buffered saline, fixed with 200 ul of 1% paraformaldehyde 1% sodium cacodilate-NaCl and kept cold until data acquisition using a FACScan. The antibodies used for the staining were anti-CD4-PE, anti-CD8-PE, anti-HLA-DR-FITC, anti-CD19-PE, and Ig-FITC and PE controls, all purchased from Immunotech (Birmingham, UK).
Single cell cytoplasmic cytokine analysis
Lymphocytes were analysed for their intracellular cytokine expression pattern as described below and by Sornaze et al[37]. Briefly, cells frozen in DMSO and normal human sera as described by Yssel [38], were brought from the endemic area in Brazil to DNAX Research Institute in Palo Alto, CA, USA (2 months following the initial collection of PBMC). Cells were thawed and stimulated in culture for 7 hours with a combination of anti-CD3 and anti-CD28 antibodies in presence of IL-2 to reveal their ex vivo cytokine profile. Cultures were exposed to Brefeldin for a period of at least 4 hours. The cells were then fixed using formaldehyde, permeabilized with a solution of saponin and stained using anti-cytokine mAbs directly conjugated with either FITC (IFN-γ and IL-2) or PE (IL-4 and IL-5) using the pairs of IFN-γ and IL-4 or IL-2 and IL-5 for 30 minutes at 4°C. Preparations were washed and fixed as described in the previous section and analyzed in a FACScalibur, selecting the lymphocyte population. 30,000.00 events were counted. FITC and PE-labeled Ig control antibodies as well as a control of unstimulated PBMC were also included in all experiments. In addition, the cells where submitted to a confirmatory analysis using the same surface markers used on the fresh lymphocytes taken from the individuals (see above). This analysis did not show significant differences in the percent of CD4+, CD8+, or CD19+ cells as compared to the before freezing analysis. A recovery of 70% of the cells, on average, was achieved using the slow freezing and quick thaw method described by Yssel [38].
Author contributions
This information is being supplied as a requirement of the editors, and is not meant to diminish the contribution of any given individual to the completion of the work, which would have been impossible without the efforts of all involved (KJG). All authors contributed greatly to the completion of the above mentioned work. Author 1 (WOD) executed immunology experiments along with Authors 4 (LFC) and 10 (KJG), conceived the experimental design along with Authors 3 (DD) and 10 (KJG), and produced the first draft of the manuscript. Authors 2 (RCO), 5 (LF), and 6 (AMSS) were critical for obtaining blood samples from schistosomiasis patients and access to the endemic area, as well as for research facilities (ROC). Authors 3 (DD) and 8 (MW) were responsible for patient identification and classification, as well as epidemiology. Author 9 (HY) provided the facilities, technical training, and advise with experimental design related to intracellular cytoplasmic staining of cytokines at DNAX Research Institute. Author 10 (KJG) conceived the original experimental design, implemented immunology experiments, and elaboration of the manuscript along with Author 1 (WOD).
Acknowledgments
Acknowledgements
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), PRONEX (Brazilian Research Financing Agency), and CNPq (Brazilian Research Financing Agency supplying student fellowships). Also, significant support was given by DNAX Research Institute, Palo Alto, CA.
Contributor Information
Walderez O Dutra, Email: waldutra@mono.icb.ufmg.br.
Rodrigo Correa-Oliveira, Email: correa@cpqrr.fiocruz.br.
David Dunne, Email: dd@mole.bio.cam.ac.uk.
Luiza Fosenca Cecchini, Email: Luizafc@aol.com.
Lúcia Fraga, Email: correa@cpqrr.fiocruz.br.
Morven Roberts, Email: dd@mole.bio.cam.ac.uk.
Alda Maria Soares-Silveira, Email: correa@cpqrr.fiocruz.br.
Michelle Webster, Email: dd@mole.bio.cam.ac.uk.
Hans Yssel, Email: yssel@montp.inserm.fr.
Kenneth J Gollob, Email: kjgollob@mono.icb.ufmg.br.
References
- Todd CW, Colley DG, Ramzy RM, Habib M, Alamy El MA. Comparison of whole blood cultures and peripheral blood mononuclear cell cultures for evaluation of lymphocyte reactivity during chronic schistosomiasis. Trans R Soc Trop Med Hyg. 1981;75:783–7. doi: 10.1016/0035-9203(81)90412-0. [DOI] [PubMed] [Google Scholar]
- Colley DG, Garcia AA, Lambertucci JR, Parra JC, Katz N, Rocha RS, Gazzinelli G. Immune responses during human schistosomiasis. XII. Differential responsiveness in patients with hepatosplenic disease. Am J Trop Med Hyg. 1986;35:793–802. [PubMed] [Google Scholar]
- Butterworth AE, Dunne DW, Fulford AJ, Ouma JH, Sturrock RF. Immunity and morbidity in Schistosoma mansoni infection: quantitative aspects. Am J Trop Med Hyg. 1996;55:109–15. doi: 10.4269/ajtmh.1996.55.109. [DOI] [PubMed] [Google Scholar]
- Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–5. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Dunne DW, Webster M, Smith P, Langley JG, Richardson BA, Fulford AJ, Butterworth AE, Sturrock RF, Kariuki HC, Ouma JH. The isolation of a 22 kDa band after SDS-PAGE of Schistosoma mansoni adult worms and its use to demonstrate that IgE responses against the antigen(s) it contains are associated with human resistance to reinfection. Parasite Immunol. 1997;19:79–89. doi: 10.1046/j.1365-3024.1997.d01-186.x. [DOI] [PubMed] [Google Scholar]
- Webster M, Fallon PG, Fulford AJ, Butterworth AE, Ouma JH, Kimani G, Dunne DW. IgG4 and IgE responses to Schistosoma mansoni adult worms after treatment. J Infect Dis. 1997;175:493–4. doi: 10.1093/infdis/175.2.493. [DOI] [PubMed] [Google Scholar]
- Webster M, Roberts M, Fulford AJ, Marguerite M, Gallisot MC, Diagne M, Niang M, Riveau G, Capron A, Dunne DW. Human IgE responses to rSm22.6 are associated with infection intensity rather than age per se, in a recently established focus of Schistomiasis mansoni. Trop Med Int Health. 1998;3:318–26. doi: 10.1046/j.1365-3156.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- Webster M, Fulford AJ, Braun G, Ouma JH, Kariuki HC, Havercroft JC, Gachuhi K, Sturrock RF, Butterworth AE, Dunne DW. Human immunoglobulin E responses to a recombinant 22.6-kilodalton antigen from Schistosoma mansoni adult worms are associated with low intensities of reinfection after treatment. Infect Immun. 1996;64:4042–6. doi: 10.1128/iai.64.10.4042-4046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana IR, Correa-Oliveira R, Carvalho O, dos S, Massara CL, Colosimo E, Colley DG, Gazzinelli G. Comparison of antibody isotype responses to Schistosoma mansoni antigens by infected and putative resistant individuals living in an endemic area. Parasite Immunol. 1995;17:297–304. doi: 10.1111/j.1365-3024.1995.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Punnonen J, Yssel H, de Vries JE. The relative contribution of IL-4 and IL-13 to human IgE synthesis induced by activated CD4+ or CD8+ T cells. J Allergy Clin Immunol. 1997;100:792–801. doi: 10.1016/s0091-6749(97)70276-8. [DOI] [PubMed] [Google Scholar]
- Gounni AS, Lamkhioued B, Delaporte E, Dubost A, Kinet JP, Capron A, Capron M. The high-affinity IgE receptor on eosinophils: from allergy to parasites or from parasites to allergy? J Allergy Clin Immunol. 1994;94:1214–6. doi: 10.1016/0091-6749(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Capron M. Eosinophils and parasites. Ann Parasitol Hum Comp. 1991;66 Suppl 1:41–5. [PubMed] [Google Scholar]
- Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–85. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CL, Xianli J, Malhotra I, Liu S, Mahmoud AA, Oettgen HC. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J Immunol. 1997;158:294–300. [PubMed] [Google Scholar]
- Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920–30. [PubMed] [Google Scholar]
- Yssel H, Groux H. Characterization of T cell subpopulations involved in the pathogenesis of asthma and allergic diseases. Int Arch Allergy Immunol. 2000;121:10–8. doi: 10.1159/000024292. [DOI] [PubMed] [Google Scholar]
- Gazzinelli G, Katz N, Rocha RS, Colley DG. Immune responses during human schistosomiasis mansoni. X. Production and standardization of an antigen-induced mitogenic activity by peripheral blood mononuclear cells from treated, but not active cases of schistosomiasis. J Immunol. 1983;130:2891–5. [PubMed] [Google Scholar]
- de Jesus AM, Almeida RP, Bacellar O, Araujo MI, Demeure C, Bina JC, Dessein AJ, Carvalho EM. Correlation between cell-mediated immunity and degree of infection in subjects living in an endemic area of schistosomiasis. Eur J Immunol. 1993;23:152–8. doi: 10.1002/eji.1830230125. [DOI] [PubMed] [Google Scholar]
- Parra JC, Lima MS, Gazzinelli G, Colley DG. Immune responses during human schistosomiasis mansoni. XV. Anti-idiotypic T cells can recognize and respond to anti-SEA idiotypes directly. J Immunol. 1988;140:2401–5. [PubMed] [Google Scholar]
- Marguerite M, Gallissot MC, Diagne M, Moreau C, Diakkhate MM, Roberts M, Remoue F, Thiam A, Decam C, Rogerie F, Cottrez F, Neyrinck JL, Butterworth AE, Sturrock RF, Piau JP, Daff B, Niang M, Wolowczuk I, Riveau G, Auriault C, Capron A. Cellular immune responses of a Senegalese community recently exposed to Schistosoma mansoni: correlations of infection level with age and inflammatory cytokine production by soluble egg antigen-specific cells. Trop Med Int Health. 1999;4:530–43. doi: 10.1046/j.1365-3156.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira LM, Gazzinelli G, Eloi-Santos SM, Cunha-Melo JR, Alves-Oliveira LF, Silveira AM, Viana IR, Carmo J, Souza A, Correa-Oliveira R. Differential cellular reactivity to adult worm antigens of patients with different clinical forms of schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1992;86:57–61. doi: 10.1016/0035-9203(92)90441-e. [DOI] [PubMed] [Google Scholar]
- Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–72. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- Falcao PL, Malaquias LC, Martins-Filho OA, Silveira AM, Passos VM, Prata A, Gazzinelli G, Coffman RL, Correa-Oliveira R. Human Schistosomiasis mansoni: IL-10 modulates the in vitro granuloma formation. Parasite Immunol. 1998;20:447–54. doi: 10.1046/j.1365-3024.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- Martins-Filho OA, Dutra WO, Freeman GL, Silveira AM, Rabello A, Colley DG, Prata A, Gazzinelli G, Correa-Oliveira R, Carvalho-Parra J. Flow cytometric study of blood leucocytes in clinical forms of human schistosomiasis. Scand J Immunol. 1997;46:304–11. doi: 10.1046/j.1365-3083.1997.d01-119.x. [DOI] [PubMed] [Google Scholar]
- Gollob KJ, Coffman RL. A minority subpopulation of CD4+ T cells directs the development of naive CD4+ T cells into IL-4-secreting cells. J Immunol. 1994;152:5180–8. [PubMed] [Google Scholar]
- Dutra WO, Martins-Filho OA, Cancado JR, Pinto-Dias JC, Brener Z, Freeman Junior GL, Colley DG, Gazzinelli G, Parra JC. Activated T and B lymphocytes in peripheral blood of patients with Chagas' disease. Int Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- Dutra WO, Gollob KJ, Pinto-Dias JC, Gazzinelli G, Correa-Oliveira R, Coffman RL, Carvalho-Parra JF. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- Naus CW, Kimani G, Ouma JH, Fulford AJ, Webster M, van Dam GJ, Deelder AM, Butterworth AE, Dunne DW. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect Immun. 1999;67:3444–51. doi: 10.1128/iai.67.7.3444-3451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu H, Chen S, Hu L, Xie Z, Qiu Y, Su C, Cao JP, Wu Y, Zhang S, Wu G. Association between IgE antibody against soluble egg antigen and resistance to reinfection with Schistosoma japonicum. Trans R Soc Trop Med Hyg. 1997;91:606–8. doi: 10.1016/s0035-9203(97)90047-x. [DOI] [PubMed] [Google Scholar]
- Grogan JL, Kremsner PG, van Dam GJ, Metzger W, Mordmuller B, Deelder AM, Yazdanbakhsh M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–7. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- Schuerwegh AJ, De Clerck LS, De Schutter L, Bridts CH, Verbruggen A, Stevens WJ. Flow cytometric detection of type 1 (IL-2, IΦN-γ amma) and type 2 (IL-4, IL-5) cytokines in T-helper and T-suppressor/cytotoxic cells in rheumatoid arthritis, allergic asthma and atopic dermatitis. Cytokine. 1999;11:783–8. doi: 10.1006/cyto.1998.0483. [DOI] [PubMed] [Google Scholar]
- Jason J, larned J. Single-cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/S0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- Gollob KJ, Dutra WO, Coffman RL. Early message expression of interleukin-4 and interferon-gamma, but not of interleukin-2 and interleukin-10, reflects later polarization of primary CD4+ T cell cultures. Eur J Immunol. 1996;26:1565–70. doi: 10.1002/eji.1830260724. [DOI] [PubMed] [Google Scholar]
- Mahanty S, Abrams JS, King CL, Limaye AP, Nutman TB. Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol. 1992;148:3567–3571. [PubMed] [Google Scholar]
- Webster M, Correa-Oliveira R, Gazzinelli G, Viana IR, Fraga LA, Silveira AM, Dunne DW. Factors affecting high and low human IgE responses to schistosome worm antigens in an area of Brazil endemic for Schistosoma mansoni and hookworm. Am J Trop Med Hyg. 1997;57:487–94. doi: 10.4269/ajtmh.1997.57.487. [DOI] [PubMed] [Google Scholar]
- Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yssel H, Spits H. Generation and maintenance of cloned human T cell lines. In Current Protocols in Immunology, John Wiley & Sons, New York. 2002;Unit 7.19 doi: 10.1002/0471142735.im0719s47. [DOI] [PubMed] [Google Scholar]


