Abstract
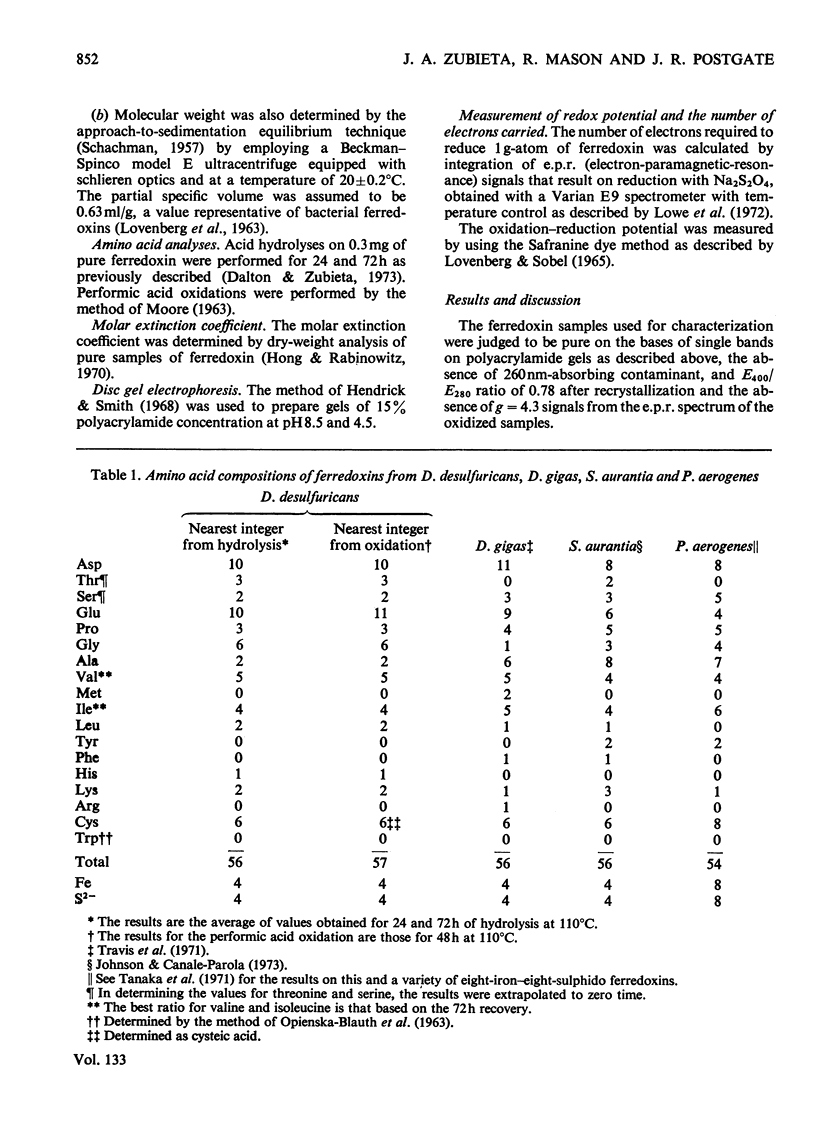
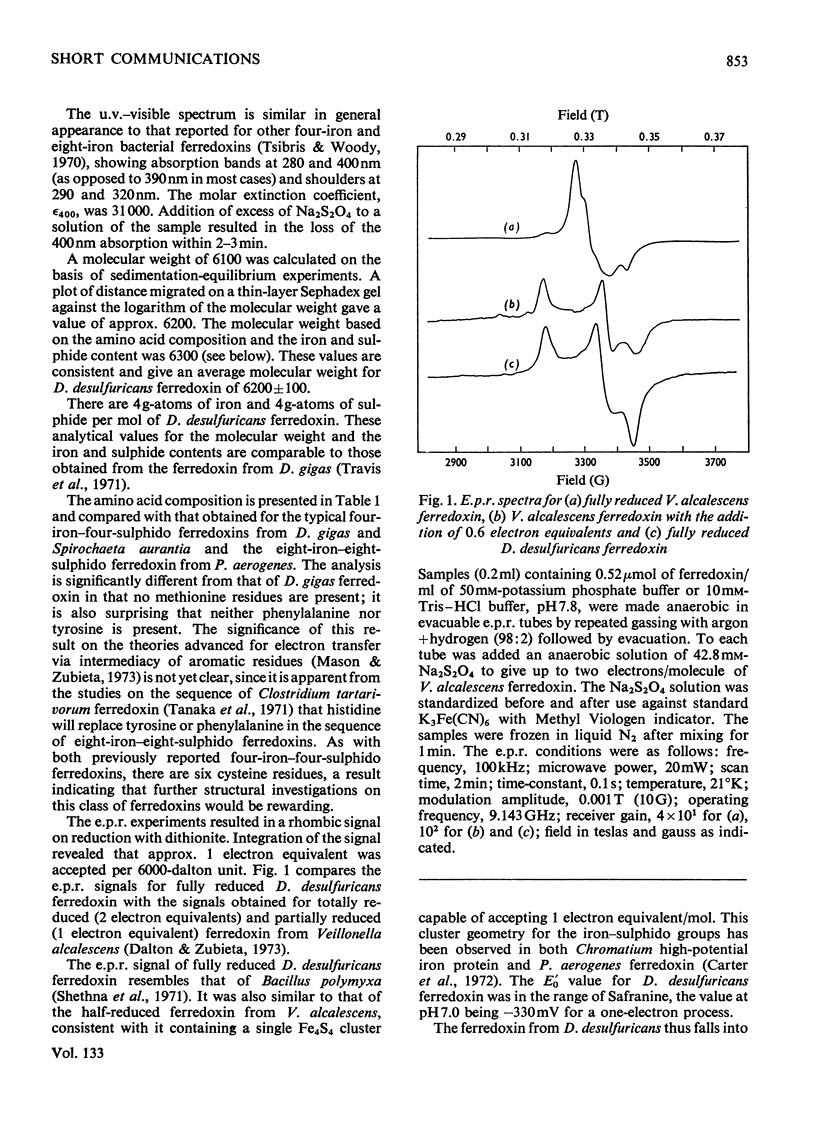
Ferredoxin from Desulfovibrio desulfuricans was isolated, purified and crystallized. It contains four iron atoms and four sulphido or `acid-labile' sulphur atoms for a molecule of 6000 daltons. The absorption spectrum in the u.v.–visible region and the electron-paramagnetic-resonance signals of the reduced protein are similar to those observed for other four-iron ferredoxins. The amino acid composition is different from that of Desulfovibrio gigas ferredoxin. The redox potential of −0.33V at pH7.0 was determined by dye techniques.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Garber N. Direct spectrophotometric determination of inorganic sulfide in biological materials and in other complex mixtures. Anal Biochem. 1971 Sep;43(1):129–133. doi: 10.1016/0003-2697(71)90116-3. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Cammack R., Rao K. K. Role for ferredoxins in the origin of life and biological evolution. Nature. 1971 Sep 10;233(5315):136–138. doi: 10.1038/233136a0. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. Molar extinction coefficient and iron and sulfide content of clostridial ferredoxin. J Biol Chem. 1970 Oct 10;245(19):4982–4987. [PubMed] [Google Scholar]
- Johnson P. W., Canale-Parola E. Properties of rubredoxin and ferredoxin isolated from spirochetes. Arch Mikrobiol. 1973;89(4):341–353. doi: 10.1007/BF00408901. [DOI] [PubMed] [Google Scholar]
- Klein R. M., Cronquist A. A consideration of the evolutionary and taxonomic significance of some biochemical, micromorphology, and physiological characters in the thallophytes. Q Rev Biol. 1967 Jun;42(2):105–296. [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Le Gall J., Dragoni N. Dependance of sulfite reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Apr 19;23(2):145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Sobel B. E. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1965 Jul;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R., Zubieta J. A. Iron-sulfur proteins: structural chemistry of their chromophores and related systems. Angew Chem Int Ed Engl. 1973 May;12(5):390–399. doi: 10.1002/anie.197303901. [DOI] [PubMed] [Google Scholar]
- Mitsui A. Purification and some chemical properties of crystalline Euglena ferredoxin. Biochim Biophys Acta. 1971 Sep 28;243(3):447–456. doi: 10.1016/0005-2795(71)90013-4. [DOI] [PubMed] [Google Scholar]
- Newman D. J., Postgate J. R. Rubredoxin from a nitrogen-fixing variety of Desulfovibrio desulfuricans. Eur J Biochem. 1968 Dec;7(1):45–50. doi: 10.1111/j.1432-1033.1968.tb19571.x. [DOI] [PubMed] [Google Scholar]
- OPIENSKA-BLAUTH J., CHAREZINSKI M., BERBEC H. A new, rapid method of determining tryptophan. Anal Biochem. 1963 Jul;6:69–76. doi: 10.1016/0003-2697(63)90009-5. [DOI] [PubMed] [Google Scholar]
- Postgate J. R. Media for sulphur bacteria. Lab Pract. 1966 Nov;15(11):1239–1244. [PubMed] [Google Scholar]
- Shethna Y. I., Stombaugh N. A., Burris R. H. Ferredoxin from Bacillus polymyxa. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1108–1116. doi: 10.1016/0006-291x(71)90019-2. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Matsueda G., Yasunobu K. T., Himes R. H., Akagi J. M., Barnes E. M., Devanathan T. The primary structure of the Clostridium tartarivorum ferredoxin, a heat-stable ferredoxin. J Biol Chem. 1971 Jun 25;246(12):3953–3960. [PubMed] [Google Scholar]
- Travis J., Newman D. J., LeGall J., Peck H. D., Jr The amino acid sequence of ferredoxin from the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1971 Oct 15;45(2):452–458. doi: 10.1016/0006-291x(71)90840-0. [DOI] [PubMed] [Google Scholar]


