Abstract
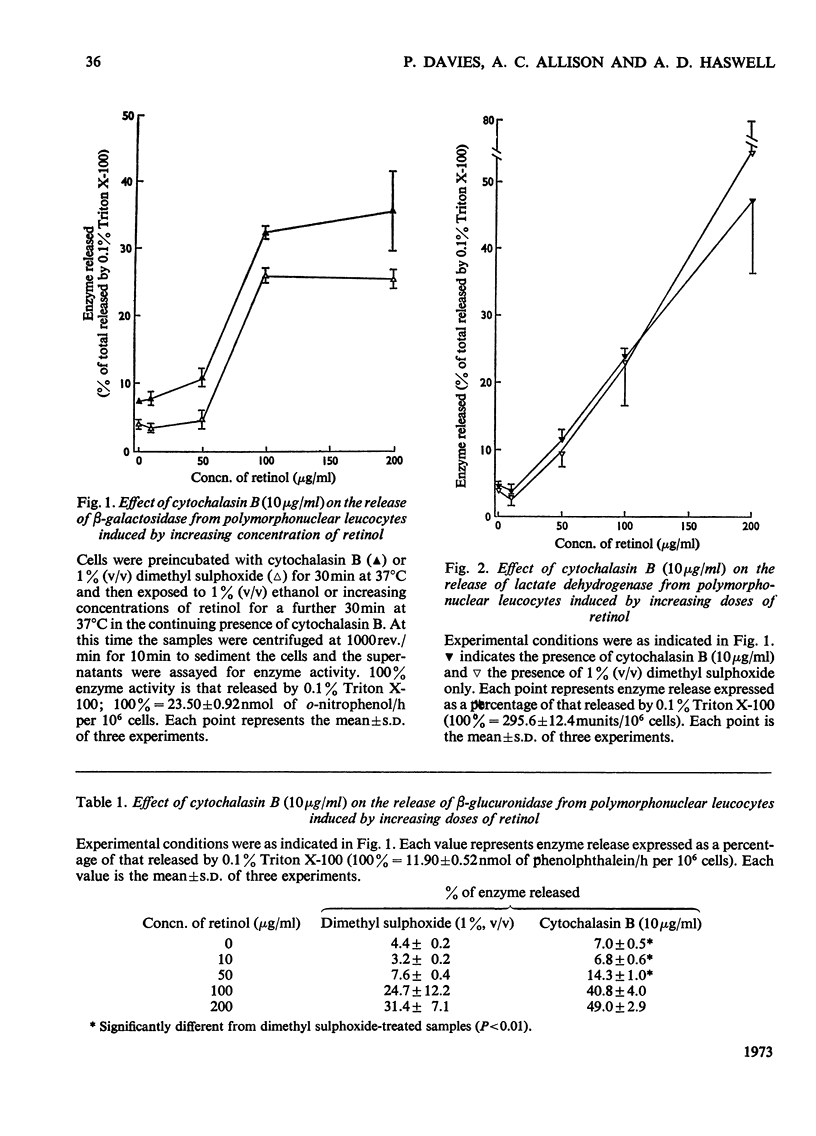
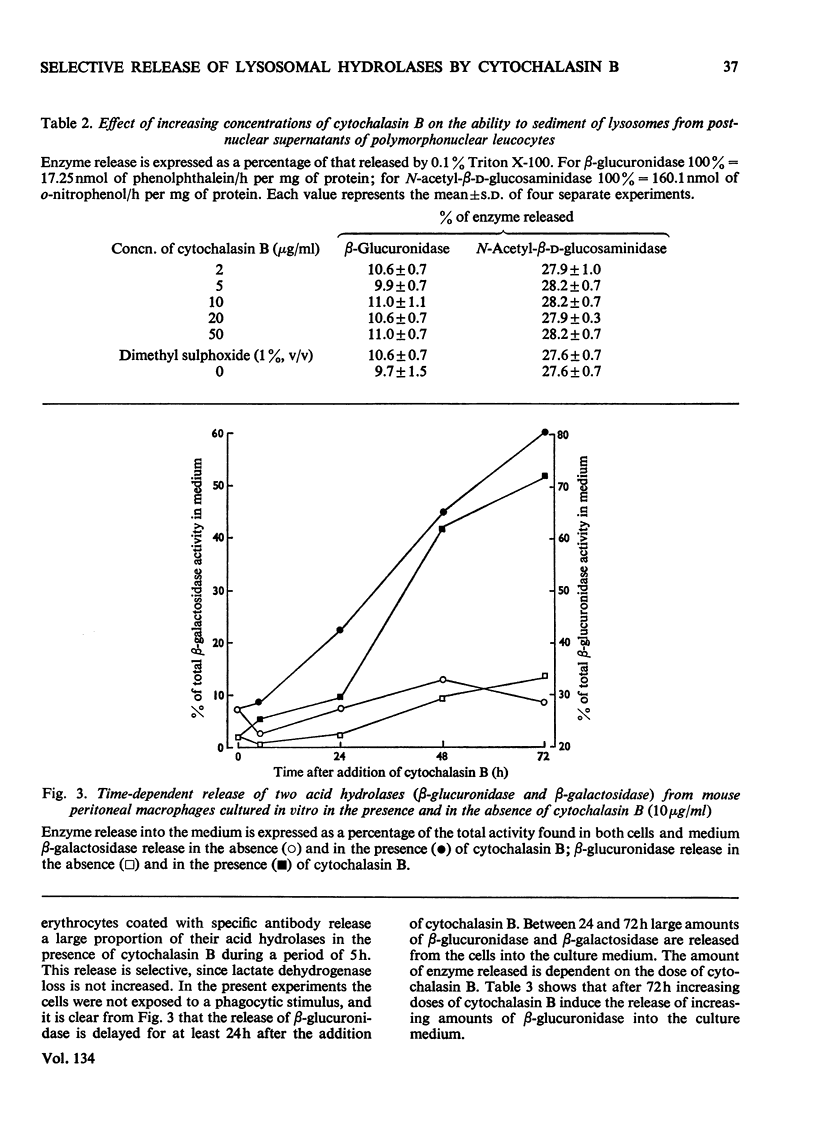
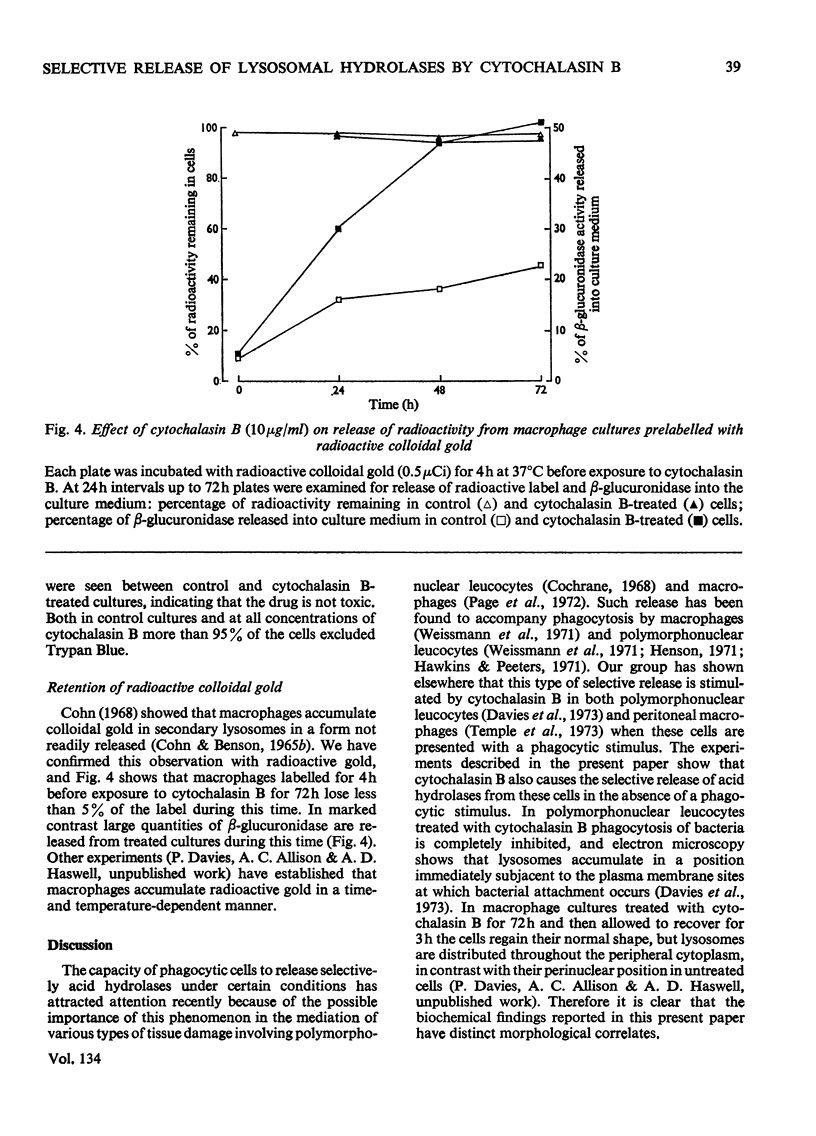
1. Cytochalasin B (10μg/ml) enhances the release of rabbit polymorphonuclear leucocyte lysosomal acid hydrolases induced by retinol (vitamin A alcohol). 2. This effect is seen at doses of the vitamin that cause selective release of acid hydrolases and those causing more general enzyme release indicated by the loss of lactate dehydrogenase. 3. Cytochalasin B (2–50μg/ml) has no effect on the release of sedimentable acid hydrolases of intact granules obtained from disrupted polymorphonuclear leucocytes. 4. Cytochalasin B (2–10μg/ml) causes a time- and dose-dependent release of mouse peritoneal macrophage acid hydrolases. 5. This effect is selective at all doses of cytochalasin B used, since no release of lactate dehydrogenase, malate dehydrogenase and leucine 2-naphthylamidase was detected. 6. Treatment with cytochalasin B at doses of up to 10μg/ml for as long as 72h did not significantly change the total activities of any of the enzymes measured. 7. The lack of toxicity of cytochalasin B was shown by dye-exclusion tests and its failure to release radioactive colloidal gold stored in secondary lysosomes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONCHIE J., FINDLAY J., LEVVY G. A. Mammalian glycosidases; distribution in the body. Biochem J. 1959 Feb;71(2):318–325. doi: 10.1042/bj0710318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A., Benson B. The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J Exp Med. 1965 Sep 1;122(3):455–466. doi: 10.1084/jem.122.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Gabbay K. H. Histamine release from human leukocytes: modulation by a cytochalasin B-sensitive barrier. J Clin Invest. 1972 Jul;51(7):1927–1931. doi: 10.1172/JCI106998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford B., Cloney R. A., Cahn R. D. Cloned pigmented retinal cells; the affects of cytochalasin B on ultrastructure and behavior. Z Zellforsch Mikrosk Anat. 1972;130(2):135–151. doi: 10.1007/BF00306953. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Krakauer K., Weissmann G. Subcellular distribution of neutral protease and peptidases in rabbit polymorphonuclear leucocytes. Nature. 1970 Nov 21;228(5273):761–762. doi: 10.1038/228761a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Rita G. A., Krakauer K., Weissmann G. Characterization of a neutral protease from lysosomes of rabbit polymorphonuclear leucocytes. Biochem J. 1971 Jul;123(4):559–569. doi: 10.1042/bj1230559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J. Uptake of biologically active substances by lysosomes. Proc R Soc Lond B Biol Sci. 1969 Apr 15;173(1030):85–93. doi: 10.1098/rspb.1969.0040. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Fell H. B., Coombs R. R. The breakdown of embryonic cartilage and bone cultivated in the presence of complement-sufficient antiserum. 2. Biochemical changes and the role of the lysosomal system. Int Arch Allergy Appl Immunol. 1967;31(3):283–303. doi: 10.1159/000229876. [DOI] [PubMed] [Google Scholar]
- Ebstensen R. D., Plagemann P. G. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1430–1434. doi: 10.1073/pnas.69.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D. Cytochalasin B. I. Effect on cytokinesis of Novikoff hepatoma cells. Proc Soc Exp Biol Med. 1971 Apr;136(4):1256–1260. doi: 10.3181/00379727-136-35470. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Ide H., Rufo R. Dual localization of acid hydrolases in endoplasmic reticulum and in lysosomes. I. Beta-glucuronidase staining reactions and cytochemical studies on kidney in androgen-stimulated mice. Histochemie. 1969;20(4):287–299. doi: 10.1007/BF00263747. [DOI] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. Cytochalasin effects on BALB-3T3 fibroblasts: dose-dependent, reversible alteration of motility and cytoplasmic cleavage. Exp Cell Res. 1971 Sep;68(1):226–228. doi: 10.1016/0014-4827(71)90610-0. [DOI] [PubMed] [Google Scholar]
- Hawkins D., Peeters S. The response of polymorphonuclear leukocytes to immune complexes in vitro. Lab Invest. 1971 Jun;24(6):483–491. [PubMed] [Google Scholar]
- Henson P. M. Interaction of cells with immune complexes: adherence, release of constituents, and tissue injury. J Exp Med. 1971 Sep 1;134(3 Pt 2):114s–135s. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F., Springer A. Cytochalasin A and B. Inhibition of sugar uptake in cultured cells. J Biol Chem. 1972 May 10;247(9):2964–2966. [PubMed] [Google Scholar]
- Krishan A. Fine structure of cytochalasin-induced multinucleated cells. J Ultrastruct Res. 1971 Jul;36(1):191–204. doi: 10.1016/s0022-5320(71)80097-7. [DOI] [PubMed] [Google Scholar]
- Krishan A., Ray-Chaudhuri R. Asynchrony of nuclear development in cytochalasin-induced multinucleate cells. J Cell Biol. 1969 Dec;43(3):618–621. doi: 10.1083/jcb.43.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Inhibition of the transport of several hexoses in mammalian cells by cytochalasin B. J Biol Chem. 1972 Jun 25;247(12):4102–4105. [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Orr T. S., Hall D. E., Allison A. C. Role of contractile microfilaments in the release of histamine from mast cells. Nature. 1972 Apr 14;236(5346):350–351. doi: 10.1038/236350a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Allison A. C. Failure of cytochalasin or colchicine to inhibit secretion of immunoglobulins. Nat New Biol. 1972 Feb 16;235(59):220–222. doi: 10.1038/newbio235220a0. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Ridler M. A., Smith G. F. The response of human cultured lymphocytes to cytochalasin B. J Cell Sci. 1968 Dec;3(4):595–602. doi: 10.1242/jcs.3.4.595. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Holtzer S., Holtzer H. Effects of cytochalasin B on muscle cells in tissue culture. Nat New Biol. 1971 Jan 27;229(4):121–123. doi: 10.1038/newbio229121a0. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. On the mechanisms of bone resorption. The action of parathyroid hormone on the excretion and synthesis of lysosomal enzymes and on the extracellular release of acid by bone cells. J Cell Biol. 1968 Dec;39(3):676–697. doi: 10.1083/jcb.39.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Wills E. J., Davies P., Allison A. C., Haswell A. D. Cytochalasin B fails to inhibit pinocytosis by macrophages. Nat New Biol. 1972 Nov 8;240(97):58–60. doi: 10.1038/newbio240058a0. [DOI] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The participation of calcium, adenosine triphosphate and adenosine triphosphatase in the extrusion of the granule proteins from the polymorphonuclear leucocyte. Biochem J. 1964 Mar;90(3):498–509. doi: 10.1042/bj0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Cytochalasin B: inhibition of D-2-deoxyglucose transport into leukocytes and fibroblasts. Science. 1972 Jun 30;176(4042):1432–1434. doi: 10.1126/science.176.4042.1432. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]


