Abstract
A soluble cytochrome was isolated and purified from the slime mould Physarum polycephalum and identified as cytochrome c by room-temperature and low-temperature (77°K) difference spectroscopy. A close similarity between P. polycephalum and mammalian cytochromes c was suggested by a comparison of the initial rates of oxidation of both proteins by mammalian mitochondria. This similarity was further emphasized by redox titrations and gel-electrophoretic studies which indicated that P. polycephalum cytochrome c has an oxidation–reduction midpoint potential of +257mV at pH7.0 and a molecular weight of 12500±1500 (mean±maximum deviation for a set of six measurements). P. polycephalum exhibits an absolute requirement for protohaemin for growth. The 59Fe-labelled haemin was prepared by chemical synthesis from protoporphyrin. The purified product had a specific radioactivity of 0.8±0.02μCi/mol. Growth of P. polycephalum in the presence of [59Fe]haemin resulted in the incorporation of 59Fe into the plasmodial cytochrome c. The specific radioactivity of the cytochrome c haem was 0.36±0.02μCi/mol. The high specific radioactivity of the cytochrome haem indicates that synthesis of the holoenzyme must proceed by direct attachment of haem to the apoprotein rather than by the intermediate formation of a protoporphyrinogen–apoprotein complex. The observed decrease in the specific radioactivity of the haem group is attributed to exchange of the 59Fe with unlabelled iron in the plasmodia either before or during attachment of the haem group to the apoprotein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES H. M., CRADDOCK V. M., SIMPSON M. V. The biosynthesis of cytochrome c in cell-free systems. I. The incorporation of labeled amino acids into cytochrome c by rat liver mitochondria. J Biol Chem. 1960 Jan;235:140–148. [PubMed] [Google Scholar]
- CHANCE B., PARSONS D. F., WILLIAMS G. R. CYTOCHROME CONTENT OF MITOCHONDRIA STRIPPED OF INNER MEMBRANE STRUCTURE. Science. 1964 Jan 10;143(3602):136–139. doi: 10.1126/science.143.3602.136. [DOI] [PubMed] [Google Scholar]
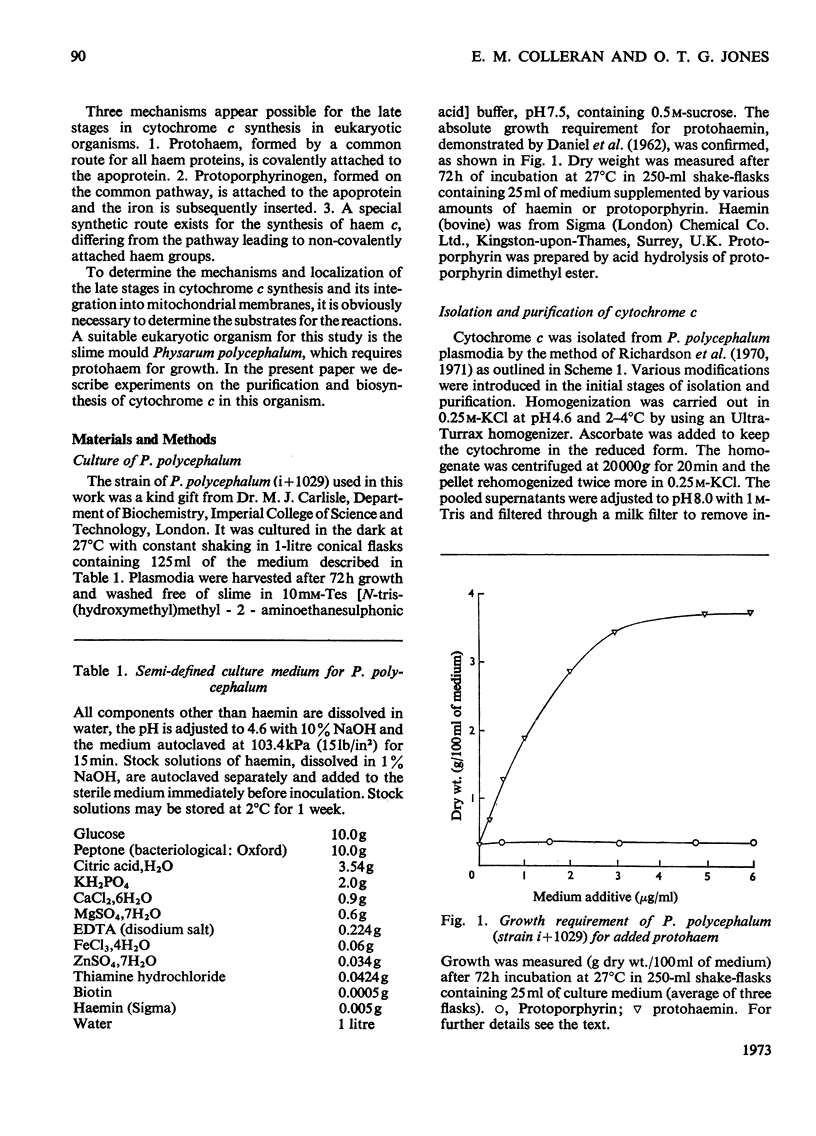
- DANIEL J. W., KELLEY J., RUSCH H. P. Hematin--requiring plasmodial myxomycete. J Bacteriol. 1962 Nov;84:1104–1110. doi: 10.1128/jb.84.5.1104-1110.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidian N., Penniall R., Elliott W. B. Origin of mitochondrial enzymes. 3. Distribution and synthesis of cytochrome c in rat liver tissue. Arch Biochem Biophys. 1969 Sep;133(2):345–358. doi: 10.1016/0003-9861(69)90463-9. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Freeman K. B., Haldar D., Work T. S. The morphological site of synthesis of cytochrome c in mammalian cells (Krebs cells). Biochem J. 1967 Dec;105(3):947–952. doi: 10.1042/bj1050947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cadavid N. F., Campbell P. N. The biosynthesis of cytochrome c. Sequence of incorporation in vivo of [14C]lysine into cytochrome c and total proteins of rat-liver subcellular fractions. Biochem J. 1967 Nov;105(2):443–450. doi: 10.1042/bj1050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Fukui S. Cytochrome C552 in Agrobacterium tumefaciens. J Biochem. 1968 Jun;63(6):780–788. doi: 10.1093/oxfordjournals.jbchem.a128843. [DOI] [PubMed] [Google Scholar]
- Kadenbach B. A quantitative study of the biosynthesis of cytochrome c. Eur J Biochem. 1969 Sep;10(2):312–317. doi: 10.1111/j.1432-1033.1969.tb00691.x. [DOI] [PubMed] [Google Scholar]
- Kadenbach B. Biosynthesis of cytochrome c. The sites of synthesis of apoprotein and holoenzyme. Eur J Biochem. 1970 Feb;12(2):392–398. doi: 10.1111/j.1432-1033.1970.tb00864.x. [DOI] [PubMed] [Google Scholar]
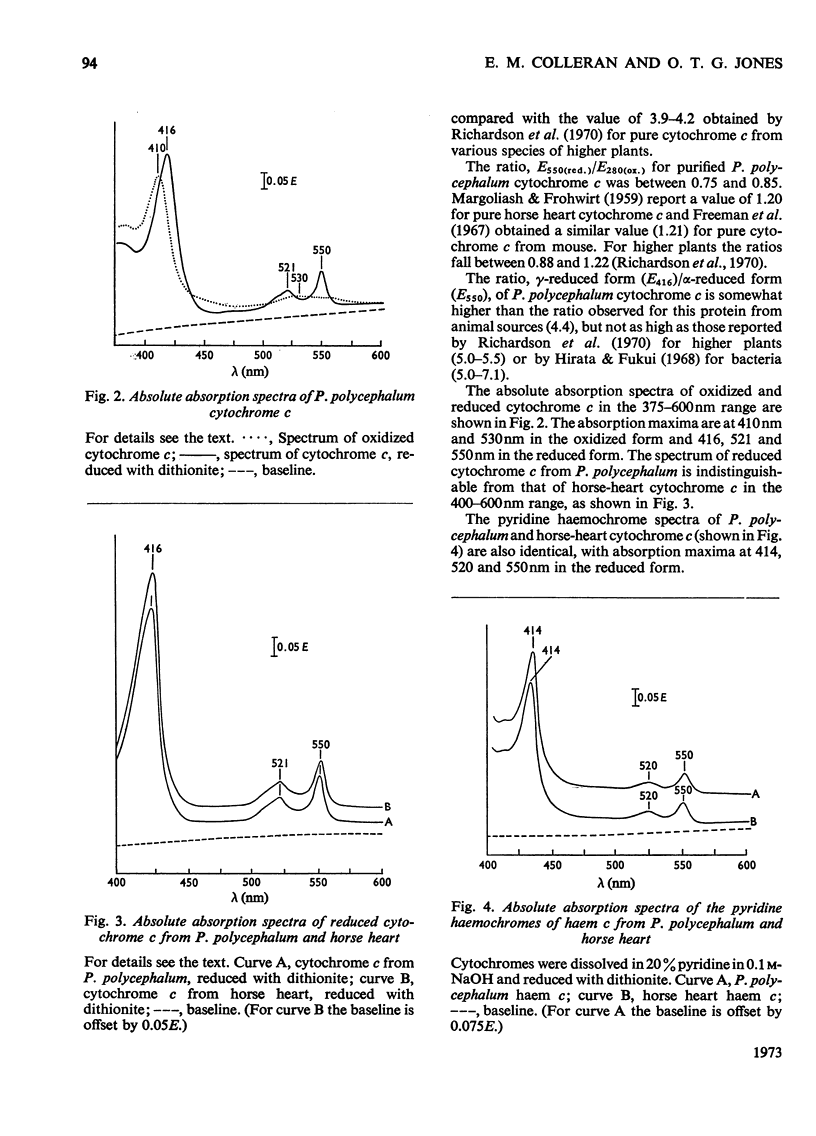
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. J., Blomquist J. C., Rusch H. P. Isolation and Characterization of an Extracellular Polysaccharide from Physarum polycephalum. J Bacteriol. 1970 Dec;104(3):1110–1118. doi: 10.1128/jb.104.3.1110-1118.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniall R., Davidian N. Origin of mitochondrial enzymes I. Cytochrome c synthesis by endoplasmic reticulum. FEBS Lett. 1968 Jul;1(1):38–41. doi: 10.1016/0014-5793(68)80013-4. [DOI] [PubMed] [Google Scholar]
- Richardson M., Richardson D., Ramshaw J. A., Thompson E. W., Boulter D. An improved method for the purification of cytochrome c from higher plants. J Biochem. 1971 Apr;69(4):811–813. doi: 10.1093/oxfordjournals.jbchem.a129530. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Gray W. R. The isolation and functional identification of a protein from the human erythrocyte 'ghost'. Biochem J. 1971 Dec;125(4):1109–1117. doi: 10.1042/bj1251109. [DOI] [PMC free article] [PubMed] [Google Scholar]


