Abstract
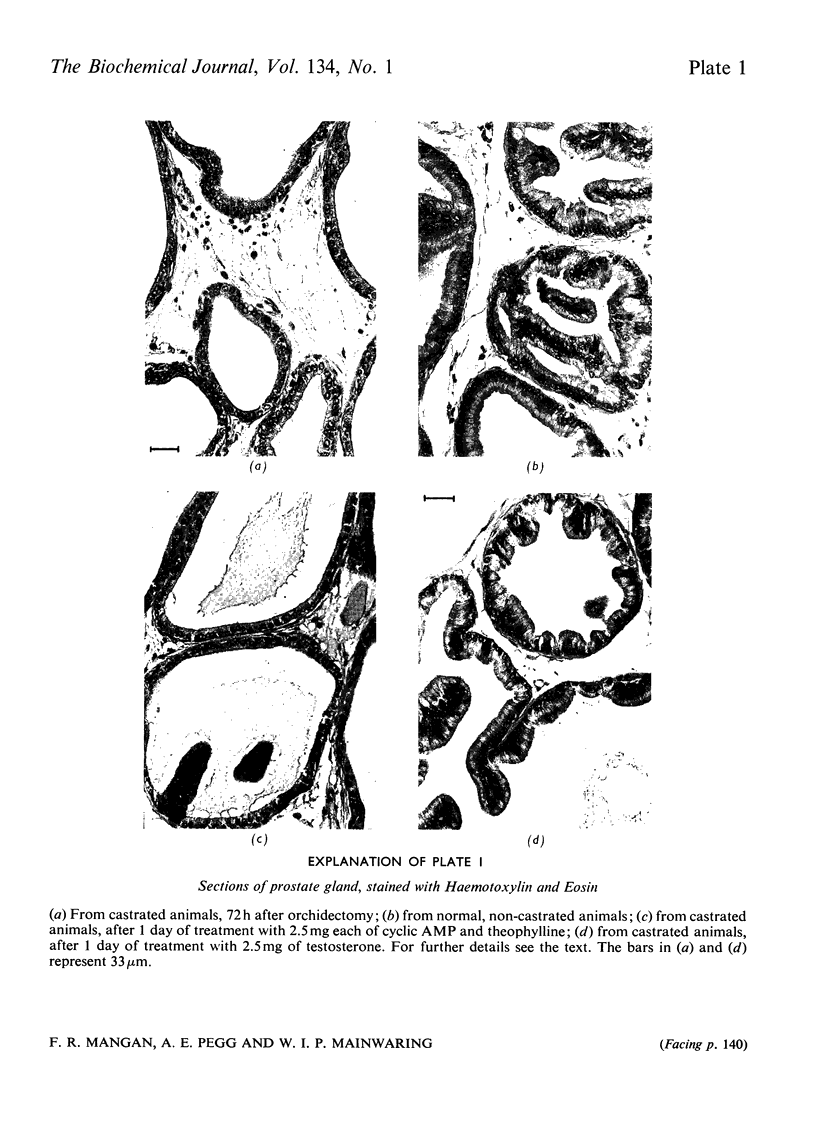
1. A comparison was made of the binding of 5α-dihydrotestosterone (17β-hydroxy-5α-androstan-3-one) and cyclic AMP in the rat prostate gland. Distinct binding mechanisms exist for these compounds, and cyclic AMP cannot serve as a competitor for the 5α-dihydrotestosterone-binding sites and vice versa. In contrast with the results obtained with 5α-dihydrotestosterone, very small amounts of cyclic AMP are retained in nuclear chromatin and the overall binding of this cyclic nucleotide is not markedly affected by castration. 2. Androgenic stimulation does not lead to major increases in the adenylate cyclase activities associated with any subcellular fraction of the prostate gland. Accordingly, changes in the concentration of cyclic AMP in the prostate gland after hormonal treatment are likely to be small, but these were not measured directly. 3. When administered to whole animals in vivo, small amounts of non-degraded cyclic AMP are found in the prostate gland but sufficient to promote an activation of certain carbohydrate-metabolizing enzymes in the cell supernatant fraction. The stimulatory effects of cyclic AMP were not evident with cytoplasmic enzymes engaged in polyamine synthesis or nuclear RNA polymerases. These latter enzymes were stimulated solely by the administration of testosterone. 4. By making use of antiandrogens, a distinction can be drawn between the biochemical responses attributable to the binding of 5α-dihydrotestosterone but not of cyclic AMP. Evidence is presented to suggest that the stimulation of RNA polymerase, ornithine decarboxylase and S-adenosyl-l-methionine decarboxylase is a consequence of the selective binding of 5α-dihydrotestosterone. Only the stimulation of glucose 6-phosphate dehydrogenase can be attributed to cyclic AMP or other metabolites of testosterone. 5. Overall, this study indicates that the formation of cyclic AMP is not a major feature of the androgenic response and affects only a restricted number of biochemical processes. Certainly, cyclic AMP cannot be considered as interchangeable with testosterone and its metabolites in the control of the function of the prostate gland. This difference is additionally emphasized by the failure of cyclic AMP to restore the morphology of the prostate gland in castrated animals; morphological restoration only follows the administration of androgens.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968 Jul 20;219(5151):277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- BRANDES D., GROTH D. P. FUNCTIONAL ULTRASTRUCTURE OF RAT PROSTATIC EPITHELIUM. Natl Cancer Inst Monogr. 1963 Oct;12:47–62. [PubMed] [Google Scholar]
- BRANDES D. Histochemical and ultrastructural observations on prostatic epithelium of older rats. Lab Invest. 1963 Mar;12:290–305. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K. L., Warren J. C. Estrogen control of carbohydrate metabolism in the rat uterus: pathways of glucose metabolism. Endocrinology. 1966 Jun;78(6):1205–1212. doi: 10.1210/endo-78-6-1205. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Jung I. A prostatic cytosol receptor. Biochem Biophys Res Commun. 1970 Feb 20;38(4):599–606. doi: 10.1016/0006-291x(70)90623-6. [DOI] [PubMed] [Google Scholar]
- Belham J. E., Neal G. E. Testosterone action in the rat ventral prostate. The effects of diethylstilboestrol and cyproterone acetate on the metabolism of ( 3 H)testosterone and the retention of labelled metabolites by rat ventral prostate in vivo and in vitro. Biochem J. 1971 Nov;125(1):81–91. doi: 10.1042/bj1250081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Bär H. P., Hechter O. Adenyl cyclase assay in fat cell ghosts. Anal Biochem. 1969 Jun;29(3):476–489. doi: 10.1016/0003-2697(69)90332-7. [DOI] [PubMed] [Google Scholar]
- CAVAZOS L. F., MELAMPY R. M. Cytological effects of testosterone propionate on epithelium of rat seminal vesicles. Endocrinology. 1954 Jun;54(6):640–648. doi: 10.1210/endo-54-6-640. [DOI] [PubMed] [Google Scholar]
- Eckstein B., Villee C. A. Effect of estradiol on enzymes of carbohydrate metabolism in rat uterus. Endocrinology. 1966 Feb;78(2):409–411. doi: 10.1210/endo-78-2-409. [DOI] [PubMed] [Google Scholar]
- Fang S., Liao S. Antagonistic action of anti-androgens on the formation of a specific dihydrotestosterone-receptor protein complex in rat ventral prostate. Mol Pharmacol. 1969 Jul;5(4):428–431. [PubMed] [Google Scholar]
- Greenman D. L. Estrogenic stimulation of cytidine uptake. Steroids. 1971 Jan;17(1):17–23. doi: 10.1016/s0039-128x(71)80112-5. [DOI] [PubMed] [Google Scholar]
- Greenman D. L. Uridine uptake by rat uterus: Influence of injection route. Endocrinology. 1970 Oct;87(4):716–722. doi: 10.1210/endo-87-4-716. [DOI] [PubMed] [Google Scholar]
- Hilf R., McDonald E., Sartini J., Rector W. D., Richards A. H. Response of uterine glucose-6-phosphate dehydrogenase isoenzymes to estrogen. Endocrinology. 1972 Jul;91(1):280–286. doi: 10.1210/endo-91-1-280. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Mammalian ornithine decarboxylase: activation and alteration of physical behaviour by thiol compounds. Biochem J. 1970 Sep;119(3):595–597. doi: 10.1042/bj1190595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel J. H., Rosenfeld M. G., Chase L. R., O'Malley B. W. Response of chick oviduct adenyl cyclase to steroid hormones. Endocrinology. 1970 May;86(5):1019–1023. doi: 10.1210/endo-86-5-1019. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang M. A., Edelman I. S. Effects of aldosterone and vasopressin on adenyl cyclase activity of rat kidney. Am J Physiol. 1972 Jan;222(1):21–24. doi: 10.1152/ajplegacy.1972.222.1.21. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M., Landau R. L. Impaired ventral prostate affinity for testosterone in hypophysectomized rats. Endocrinology. 1965 Dec;77(6):1119–1125. doi: 10.1210/endo-77-6-1119. [DOI] [PubMed] [Google Scholar]
- Liao S., Leininger K. R., Sagher D., Barton R. W. Rapid effect of testosterone on ribonucleic acid polymerase activity of rat ventral prostate. Endocrinology. 1965 Oct;77(4):763–765. doi: 10.1210/endo-77-4-763. [DOI] [PubMed] [Google Scholar]
- Liao S., Lin A. H. Prostatic nuclear chromatin: an effect of testosterone on the synthesis of ribonucleic Acid rich in cytidylyl(3',5')guanosine. Proc Natl Acad Sci U S A. 1967 Feb;57(2):379–386. doi: 10.1073/pnas.57.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Lin A. H., Tymoczko J. L. Adenyl cyclase of cell nuclei isolated from rat ventral prostate. Biochim Biophys Acta. 1971;230(3):535–538. doi: 10.1016/0304-4165(71)90185-1. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Mangan F. R., Peterken B. M. Studies on the solubilized ribonucleic acid polymerase from rat ventral prostate gland. Biochem J. 1971 Jul;123(4):619–628. doi: 10.1042/bj1230619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I., Peterken B. M. A reconstituted cell-free system for the specific transfer of steroid--receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J. 1971 Nov;125(1):285–295. doi: 10.1042/bj1250285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I. The binding of (1,2-3H)testosterone within nuclei of the rat prostate. J Endocrinol. 1969 Jul;44(3):323–333. doi: 10.1677/joe.0.0440323. [DOI] [PubMed] [Google Scholar]
- Mangan F. R., Mainwaring W. I. An explanation of the antiandrogenic properties of 6 -bromo-17 -hydroxy-17 -methyl-4-oxa-5 -androstane-3-one. Steroids. 1972 Sep;20(3):331–343. doi: 10.1016/0039-128x(72)90092-x. [DOI] [PubMed] [Google Scholar]
- Mangan F. R., Neal G. E., Williams D. C. Subcellular distribution of testosterone in rat prostate and its possible relationship to nuclear ribonucleic acid synthesis. Arch Biochem Biophys. 1968 Mar 20;124(1):27–40. doi: 10.1016/0003-9861(68)90300-7. [DOI] [PubMed] [Google Scholar]
- Miller B. G., Baggett B. Effects of 17 beta-estradiol on the incorporation of pyrimidine nucleotide precursors into nucleotide pools and RNA in the mouse uterus. Endocrinology. 1972 Mar;90(3):645–656. doi: 10.1210/endo-90-3-645. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Korenman S. G. Estrogen stimulation of synthesis of specific proteins and RNA polymerase activity in the immature chick oviduct. Biochim Biophys Acta. 1967 Aug 22;145(1):204–207. doi: 10.1016/0005-2787(67)90679-x. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Kellie A. E. The effects of oestradiol on the acid-soluble nucleotides of rat uterus. Biochem J. 1970 Sep;119(2):187–191. doi: 10.1042/bj1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Rao K. N., Talwar G. P. Action of oestradiol-17 and cyclic AMP on the synthesis of RNA, phosphoproteins and phospholipids in the uterus of ovariectomized rats in vitro. J Endocrinol. 1972 Aug;54(2):215–226. doi: 10.1677/joe.0.0540215. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Sutherland E. W. Cyclic AMP and the function of eukaryotic cells: an introduction. Ann N Y Acad Sci. 1971 Dec 30;185:5–9. doi: 10.1111/j.1749-6632.1971.tb45229.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., O'Malley B. W. Steroid hormones: effects on adenyl cyclase activity and adenosine 3',5'-momophosphate in target tissues. Science. 1970 Apr 10;168(3928):253–255. doi: 10.1126/science.168.3928.253. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Putrescine and spermidine biosynthesis in the development of normal and anucleolate mutants of Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):523–527. doi: 10.1073/pnas.68.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN A. R., STUDEN S. A comparative histochemical study of the bound lipids of the prostate gland of the dog, and the ventral prostate gland of the rat. Acta Histochem. 1960 Jun 30;9:304–319. [PubMed] [Google Scholar]
- Santti R. S., Villee C. A. Hormonal control of hexokinase in male sex accessory glands. Endocrinology. 1971 Nov;89(5):1162–1170. doi: 10.1210/endo-89-5-1162. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Talwar G. P. Action of cyclic adenosine 3',5'-monophosphate in vitro on the uptake and incorporation of uridine into ribonucleic acid in ovariectomized rat uterus. J Biol Chem. 1970 Apr 10;245(7):1513–1519. [PubMed] [Google Scholar]
- Singhal R. L., Ling G. M. Metabolic control mechanisms in mammalian systems. IV. Androgenic induction of hexokinase and glucose-6-phosphate dehydrogenase in rat seminal vesicles. Can J Physiol Pharmacol. 1969 Mar;47(3):233–239. doi: 10.1139/y69-043. [DOI] [PubMed] [Google Scholar]
- Singhal R. L., Parulekar M. R., Vijayvargiya R., Robison G. A. Metabolic control mechanisms in mammalian systems. Involvement of adenosine 3':5'-cyclic monophosphate in androgen action. Biochem J. 1971 Nov;125(1):329–342. doi: 10.1042/bj1250329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. L., Valadares J. R. Metabolic control mechanisms in mammalian systems. Hormonal regulation of phosphofructokinase in the rat prostate and seminal vesicles. Biochem J. 1968 Dec;110(4):703–711. doi: 10.1042/bj1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. L., Vijayvargiya R., Ling G. M. Cyclic adenosine monophosphate: andromimetic action on seminal vesicular enzymes. Science. 1970 Apr 10;168(3928):261–263. doi: 10.1126/science.168.3928.261. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Spelsberg T. C., Glasser S. R., O'Malley B. W. Soluble complexes between steroid hormones and target-tissue receptors bind specifically to target-tissue chromatin. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1479–1482. doi: 10.1073/pnas.68.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R., Hamilton M. J., Shields D. Effects of alpha-amanitin in vivo on RNA polymerase and nuclear RNA synthesis. Nat New Biol. 1972 Aug 9;238(84):161–164. doi: 10.1038/newbio238161a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Williams-Ashman H. G. Effects of growth hormone and tri-iodothyronine on amino acid incorporation by microsomal subfractions from rat liver. Eur J Biochem. 1967 Oct;2(3):366–374. doi: 10.1111/j.1432-1033.1967.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Unhjem O., Tveter K. J., Aakvaag A. Preliminary characterization of an androgen-macromolecular complex from the rat ventral prostate. Acta Endocrinol (Copenh) 1969 Sep;62(1):153–164. doi: 10.1530/acta.0.0620153. [DOI] [PubMed] [Google Scholar]
- Wicks W. D. Regulation of hepatic enzyme synthesis by cyclic AMP. Ann N Y Acad Sci. 1971 Dec 30;185:152–165. doi: 10.1111/j.1749-6632.1971.tb45245.x. [DOI] [PubMed] [Google Scholar]