Abstract
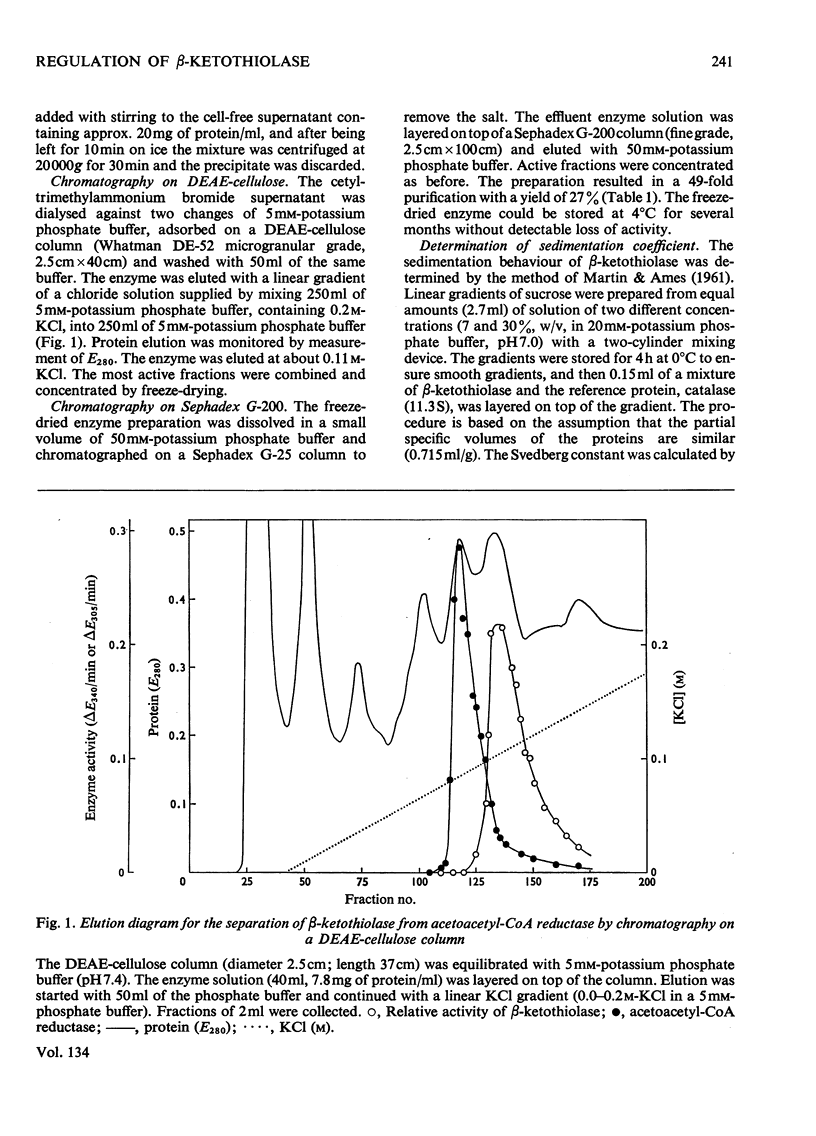
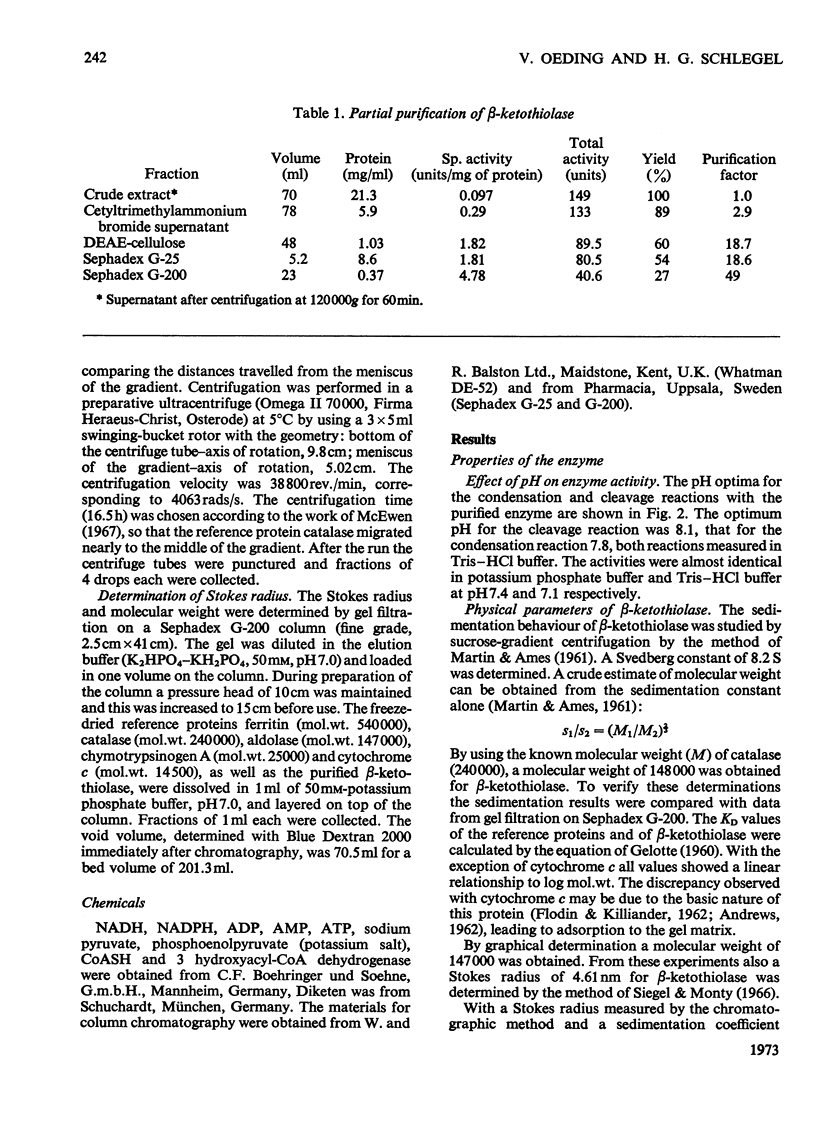
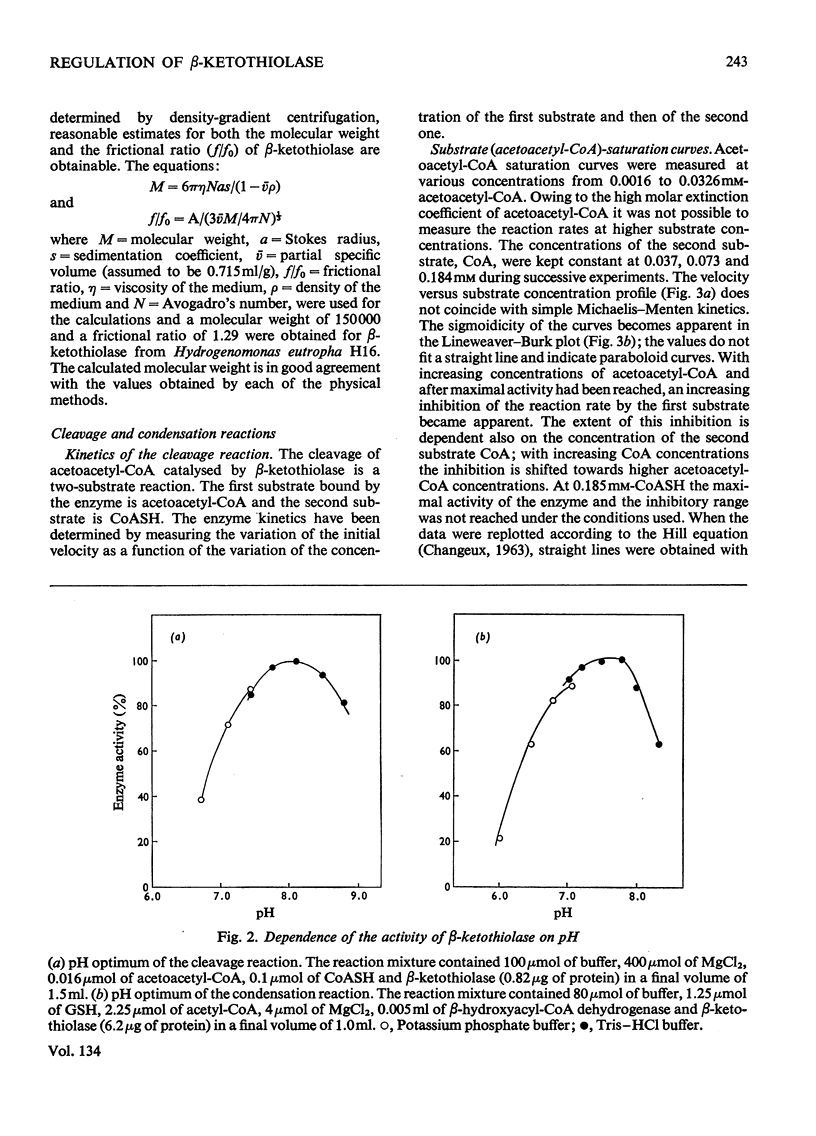
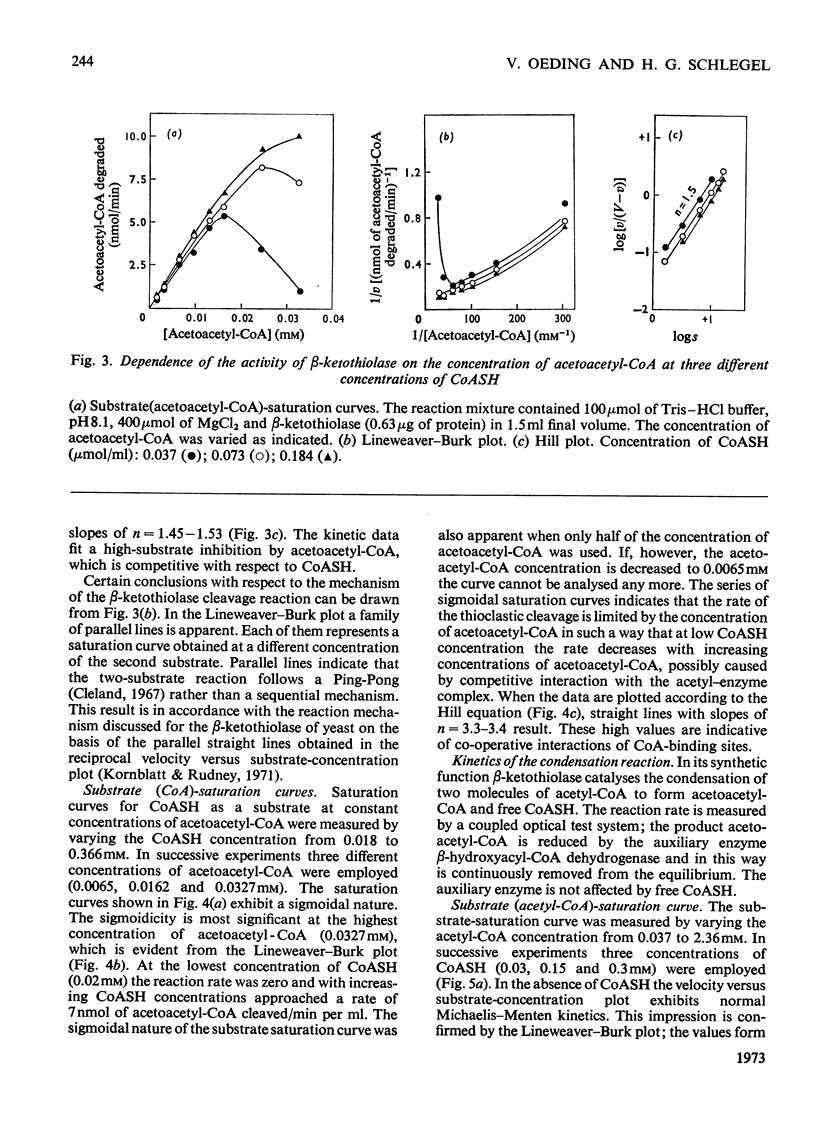
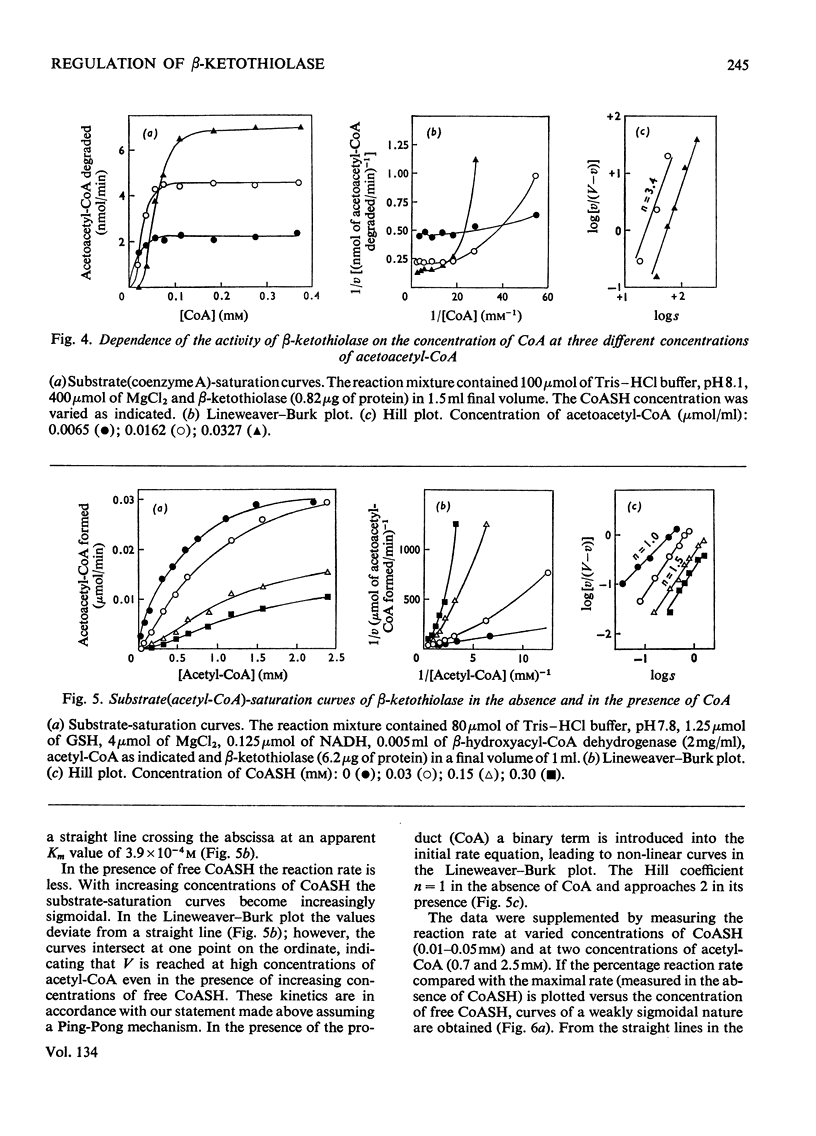
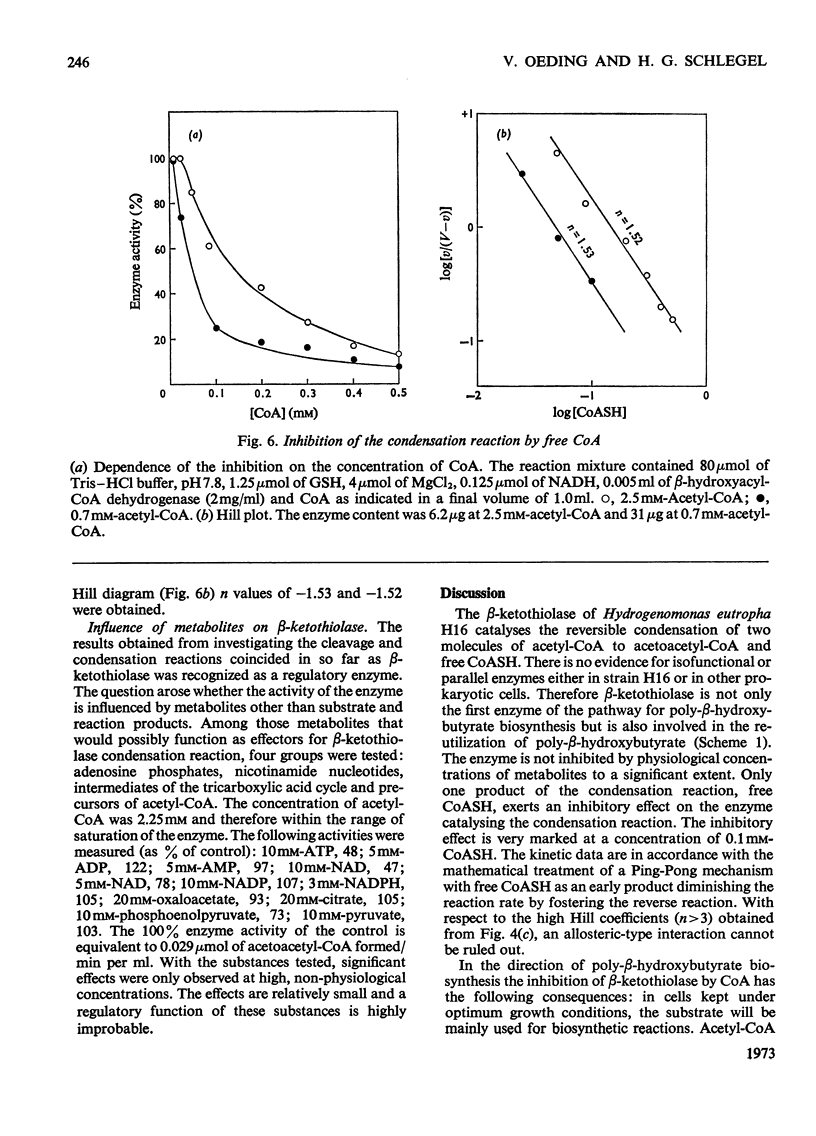
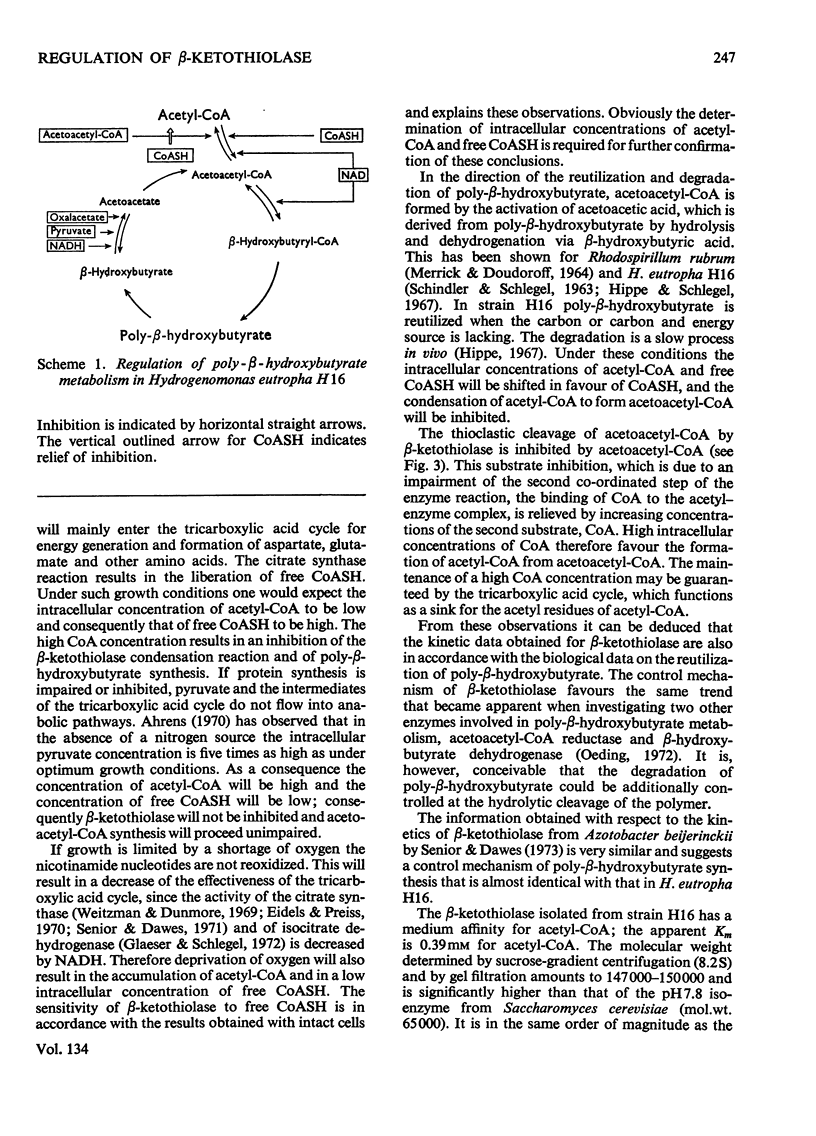
1. β-Ketothiolase was purified 49-fold from fructose-grown cells of Hydrogenomonas eutropha H16 with a yield of 27%; the purification procedure involved precipitation by cetyltrimethylammonium bromide, DEAE-cellulose chromatography and exclusion chromatography on Sephadex G-200; the freeze-dried enzyme is stable. The molecular weight determined by sucrose-gradient centrifugation (8.2S) and by gel filtration is 147000–150000. The optimum pH for the cleavage reaction is 8.1, that for the condensation reaction 7.8, both measured in Tris–HCl buffer. 2. The kinetics of the cleavage reaction are described. Substrate-saturation curves were measured with both acetoacetyl-CoA and CoA as the variable substrates. The concentration of the second substrate was kept constant and was varied during successive experiments. The cleavage reaction is characterized by substrate inhibition by acetoacetyl-CoA, which is partially relieved by free CoA. Hill plots indicate two acetoacetyl-CoA-binding sites. 3. The substrate(acetyl-CoA)-saturation curve for the condensation reaction is hyperbolic. The Km was 3.9×10−4m-acetyl-CoA. In the presence of CoA sigmoidal curves were obtained, with an increasing sigmoidicity from 0.03 to 0.30mm-CoA. The inhibitory action of CoA on the β-ketothiolase condensation reaction and its possible involvement in the regulation of poly-β-hydroxybutyrate synthesis and degradation are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS P. Estimation of molecular weights of proteins by gel filtration. Nature. 1962 Oct 6;196:36–39. doi: 10.1038/196036a0. [DOI] [PubMed] [Google Scholar]
- Eidels L., Preiss J. Citrate synthase. A regulatory enzyme from Rhodopseudomonas capsulata. J Biol Chem. 1970 Jun 10;245(11):2937–2945. [PubMed] [Google Scholar]
- Gehring U., Riepertinger C. Dissoziation und Rekonstitution der Thiolase. Eur J Biochem. 1968 Nov;6(2):281–292. doi: 10.1111/j.1432-1033.1968.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Glaeser H., Schlegel H. G. NADP- und NAD-spezifische Isocitrat-Dehydrogenase in Hydrogenomonas eutropha Stamm H 16. Arch Mikrobiol. 1972;86(4):327–337. doi: 10.1007/BF00424989. [DOI] [PubMed] [Google Scholar]
- Hippe H. Abbau und Wiederverwertung von Poly-beta-hydroxybuttersäure durch Hydrogenomonas H 16. Arch Mikrobiol. 1967 Mar 29;56(3):248–277. [PubMed] [Google Scholar]
- Hippe H., Schlegel H. G. Hydrolyse von PHBS durch intracelluläre Depolymerase von Hydrogenomonas H 16. Arch Mikrobiol. 1967 Mar 29;56(3):278–299. [PubMed] [Google Scholar]
- Kornblatt J. A., Rudney H. Two forms of acetoacetyl coenzyme A thiolase in yeast. I. Separation and properties. J Biol Chem. 1971 Jul 25;246(14):4417–4423. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. DEPOLYMERIZATION OF POLY-BETA-HYDROXYBUTYRATE BY INTRACELLULAR ENZYME SYSTEM. J Bacteriol. 1964 Jul;88:60–71. doi: 10.1128/jb.88.1.60-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei Y., Negrel R., Ailhaud G. Purification and some properties of thiolase from Escherichia coli. Biochim Biophys Acta. 1970 Oct 14;220(1):129–131. doi: 10.1016/0005-2744(70)90238-x. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- SCHINDLER J. DIE SYNTHESE VON POLY-BETA-HYDROXYBUTTERSAEURE DURCH HYDROGENOMONAS H-16 DIE ZU BETA-HYDROXYBUTYRYL-COENZYM A FUEHRENDEN REAKTIONSSCHRITTE. Arch Mikrobiol. 1964 Oct 2;49:236–255. [PubMed] [Google Scholar]
- SCHINDLER J., SCHLEGEL H. G. D-(-)-BETA-HYDROXYBUTTERSAEURE-DEHYDROGENASE AUS HYDROGENOMONAS H 16. Biochem Z. 1963 Oct 14;339:154–161. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schuster E., Schlegel H. G. Chemolithotrophes Wachstum von Hydrogenomonas H16 im Chemostaten mit elektrolytischer Knallgaserzeugung. Arch Mikrobiol. 1967;58(4):380–409. [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. Poly- -hydroxybutyrate biosynthesis and the regulation of glucose metabolism in Azotobacter beijerinckii. Biochem J. 1971 Nov;125(1):55–66. doi: 10.1042/bj1250055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The regulation of poly-beta-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J. 1973 May;134(1):225–238. doi: 10.1042/bj1340225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- WALDE E. [Studies on growth and synthesis of stored substance by Hydrogenomonas]. Arch Mikrobiol. 1962;43:109–137. [PubMed] [Google Scholar]
- Weitzman P. D., Dunmore P. Citrate synthases: allosteric regulation and molecular size. Biochim Biophys Acta. 1969 Jan 7;171(1):198–200. doi: 10.1016/0005-2744(69)90122-3. [DOI] [PubMed] [Google Scholar]


