Abstract
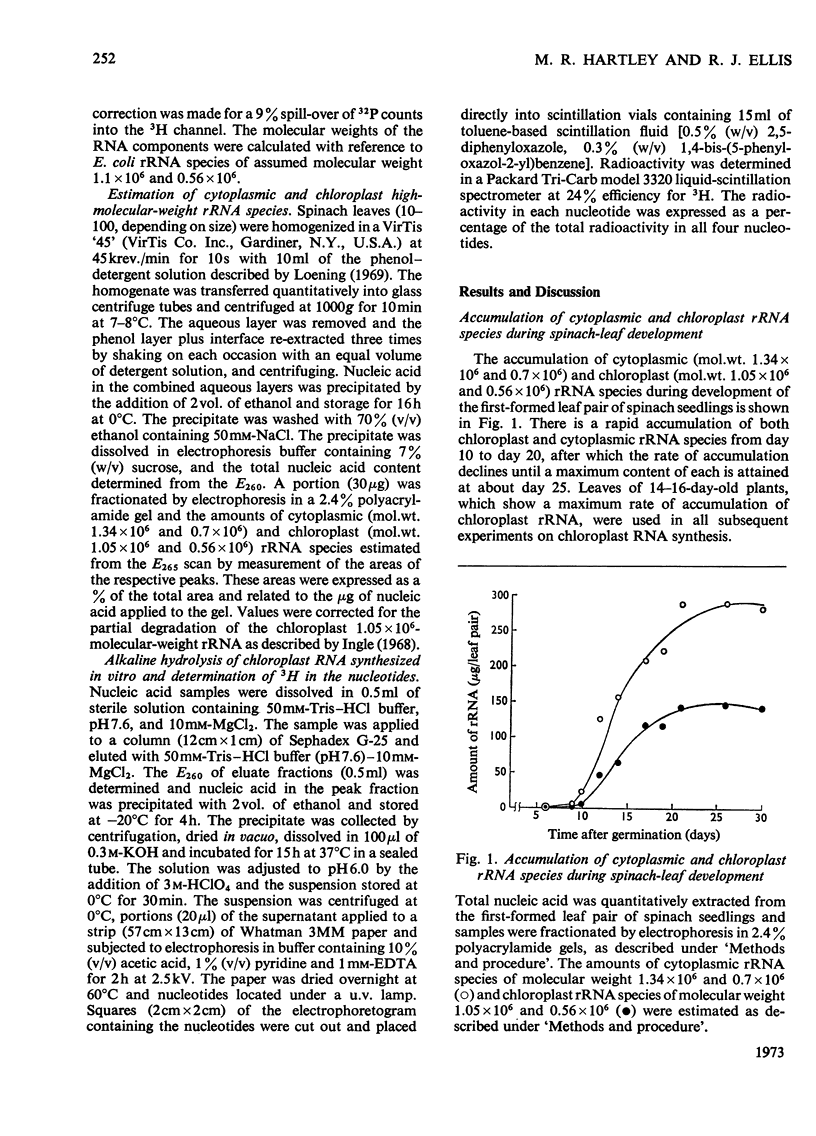
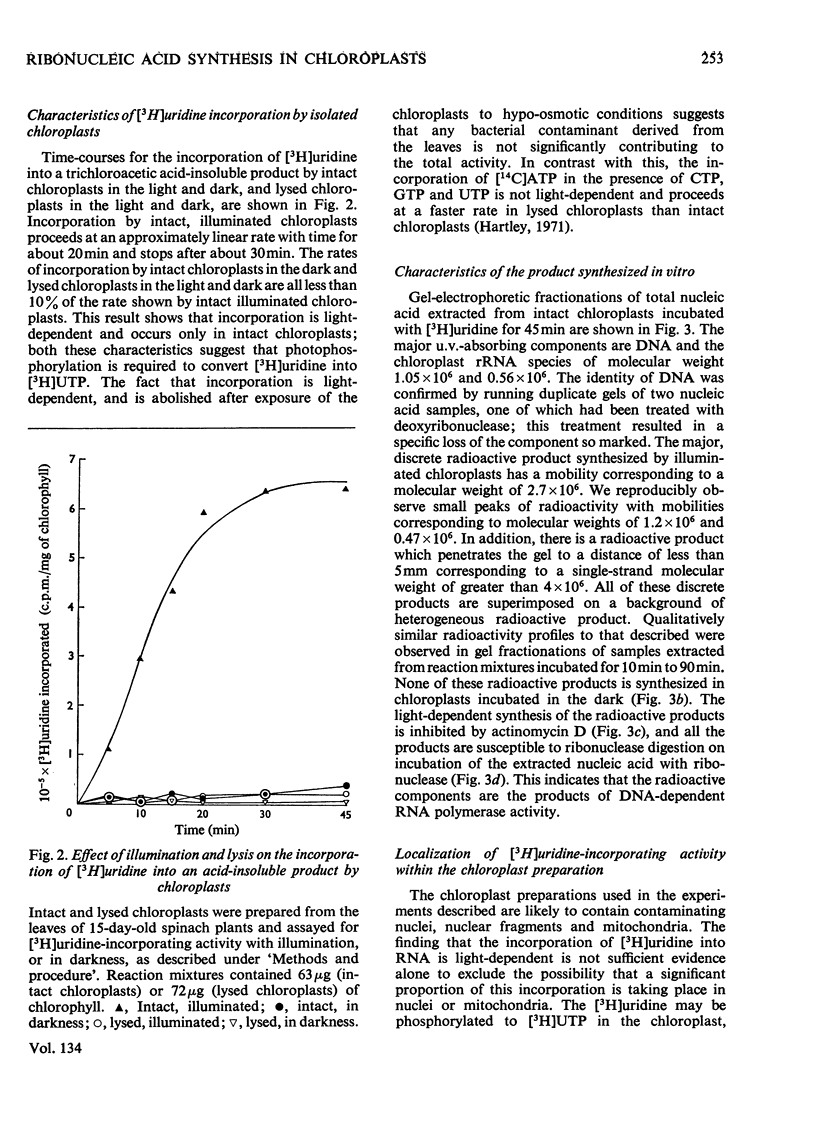
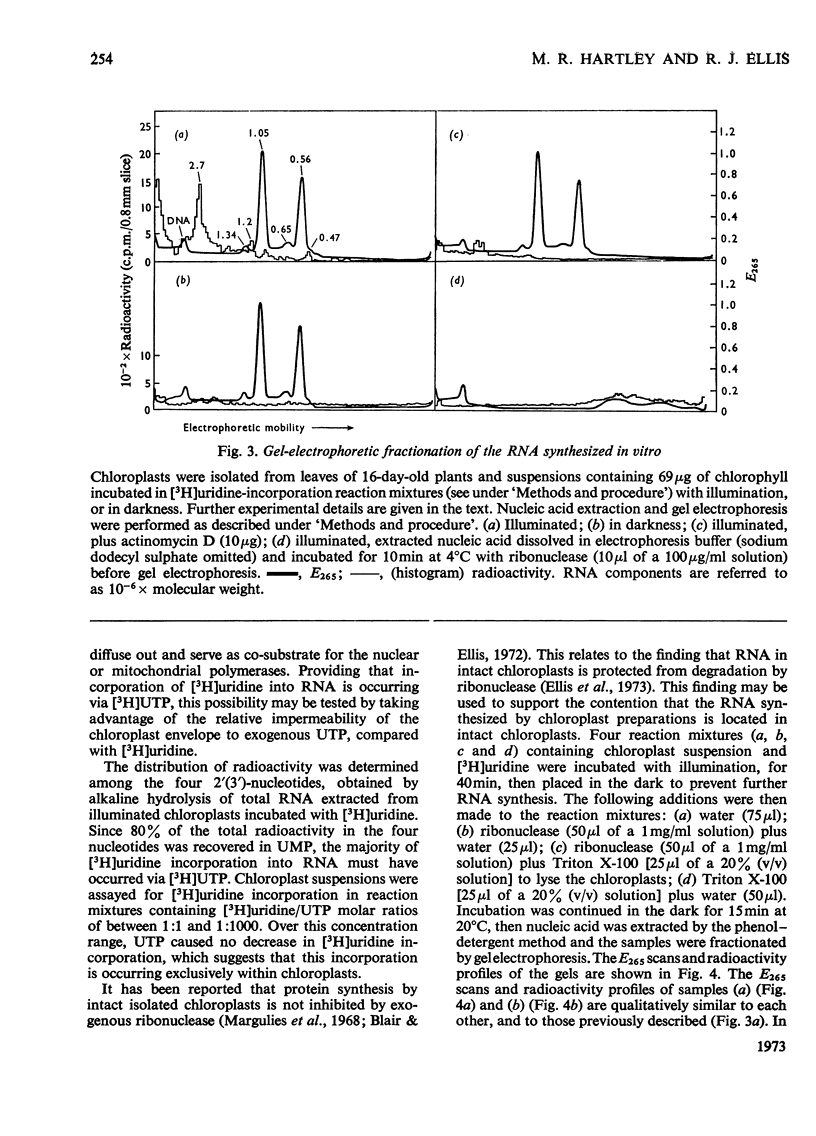
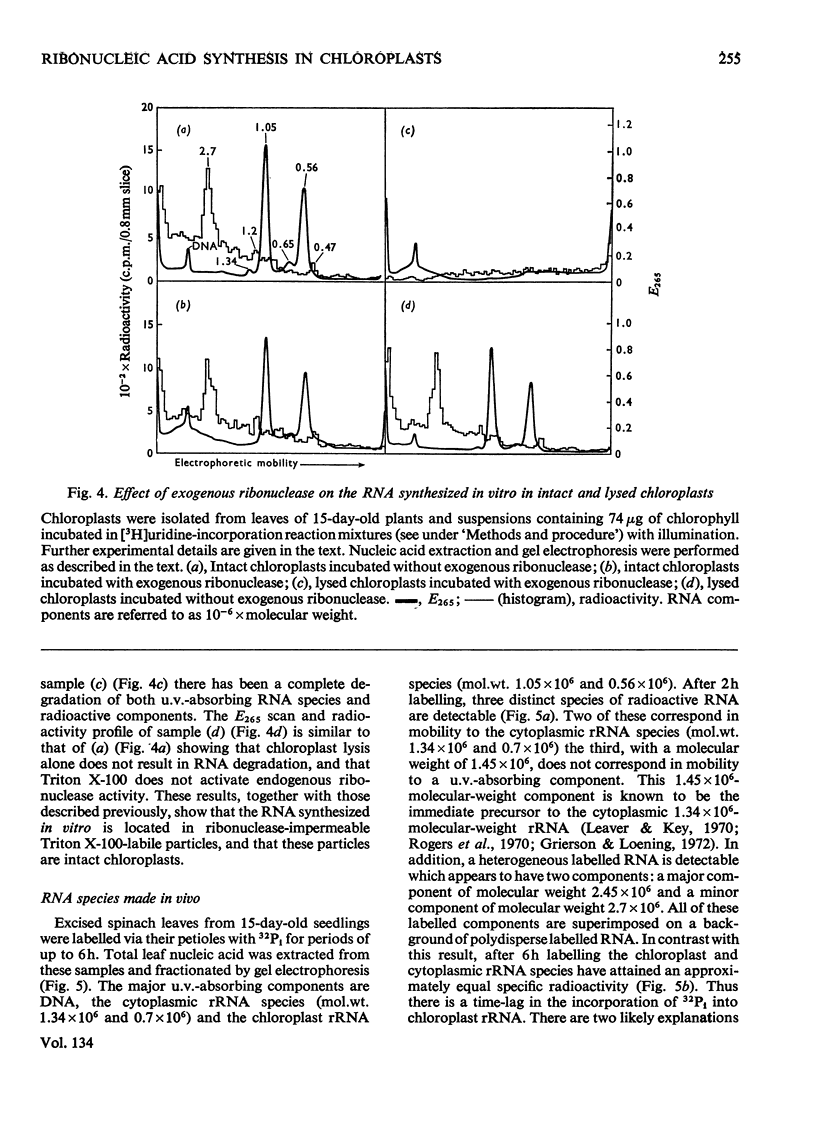
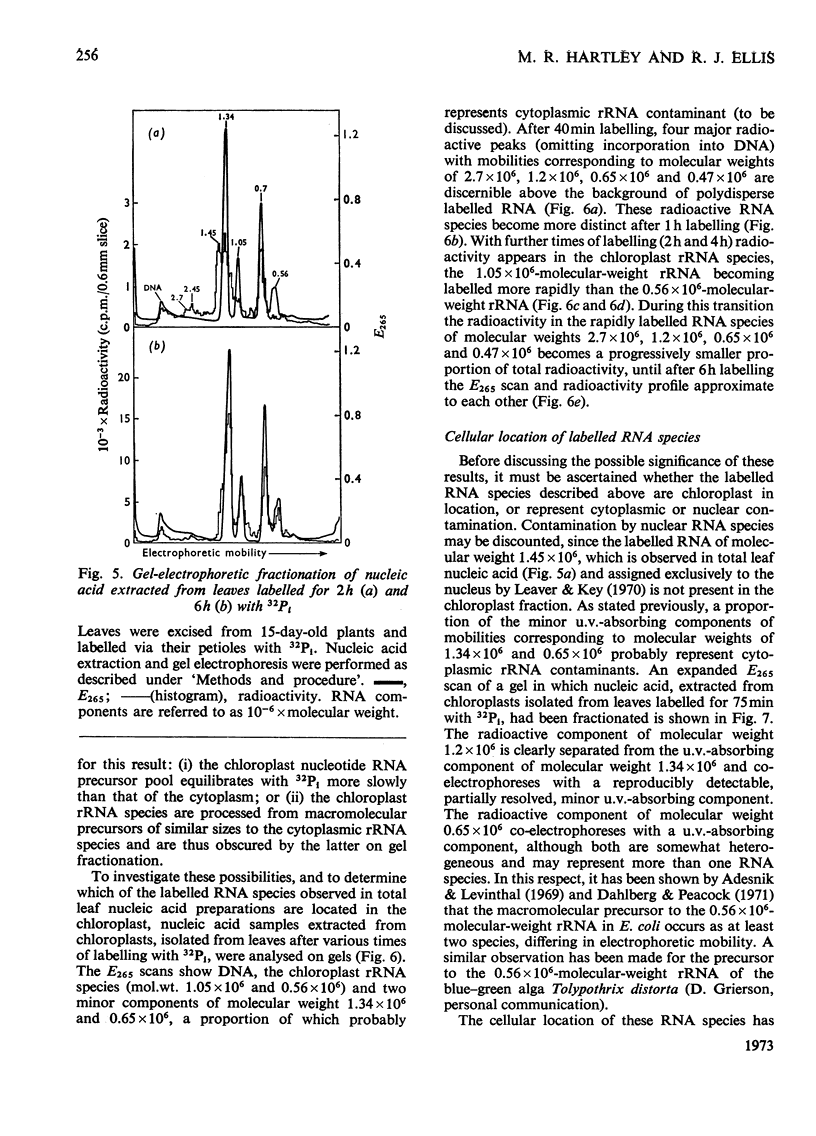
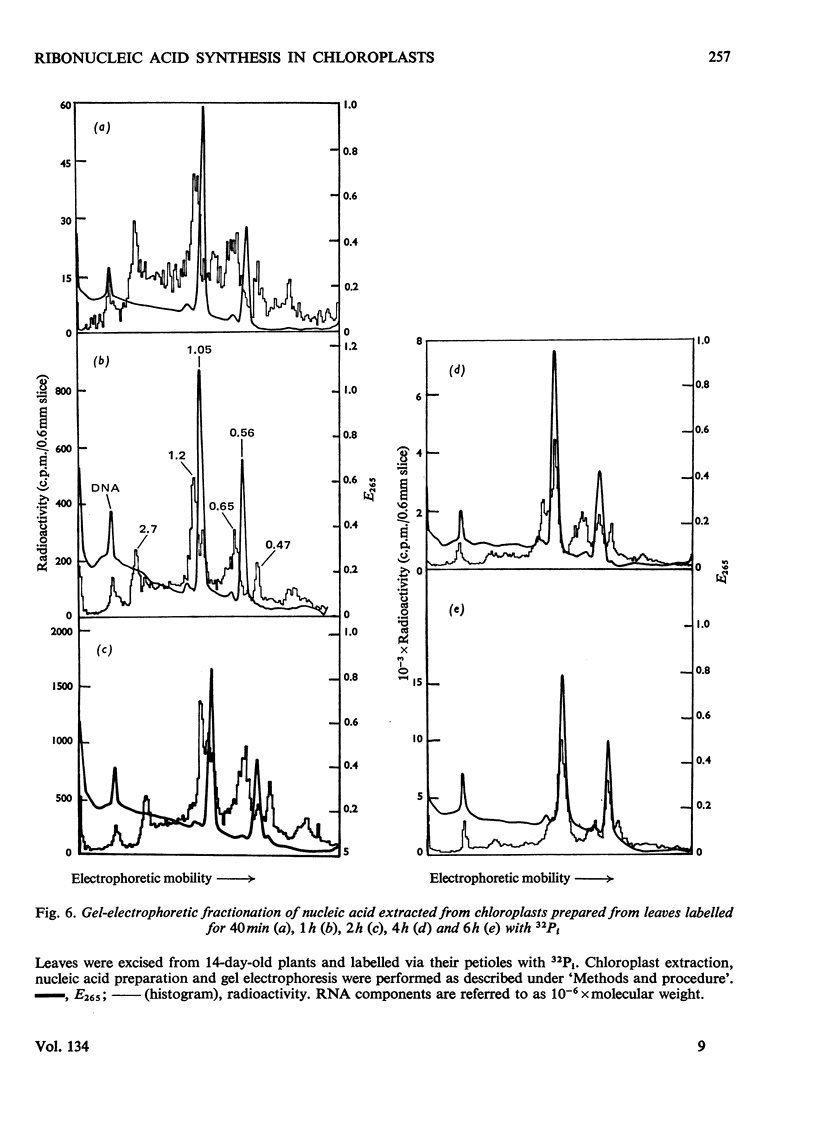
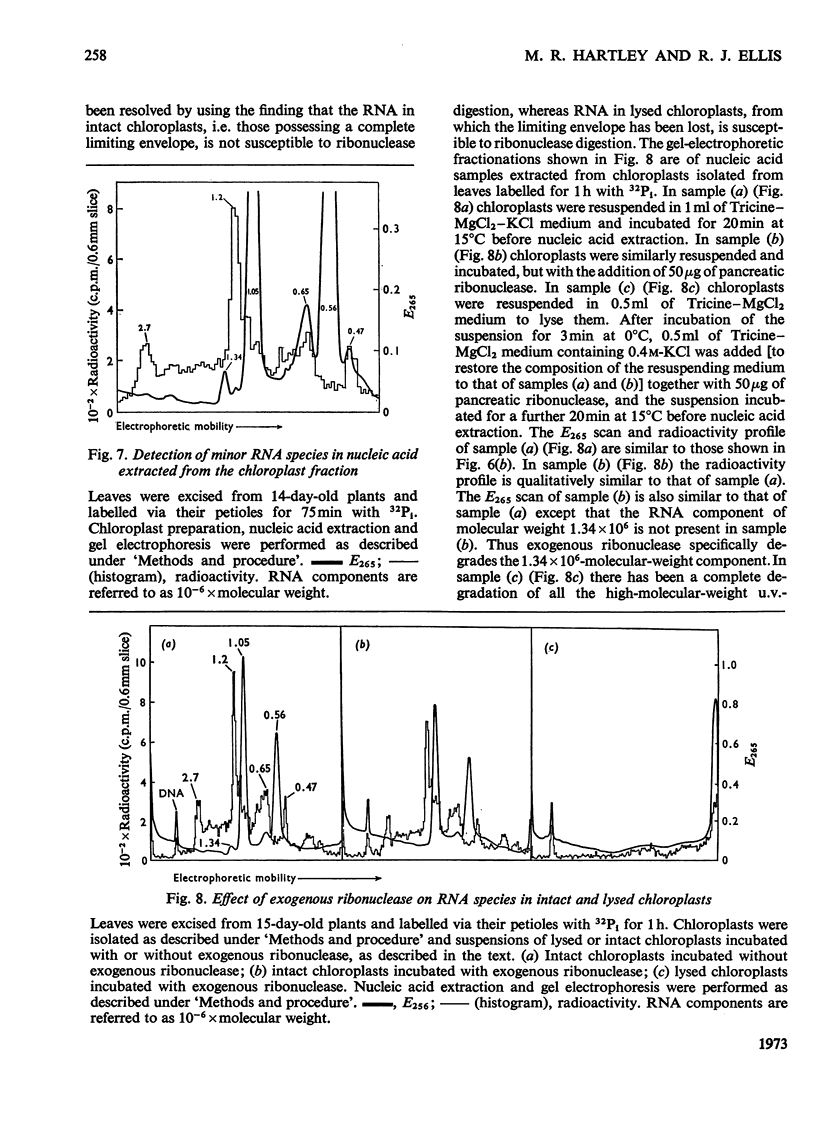
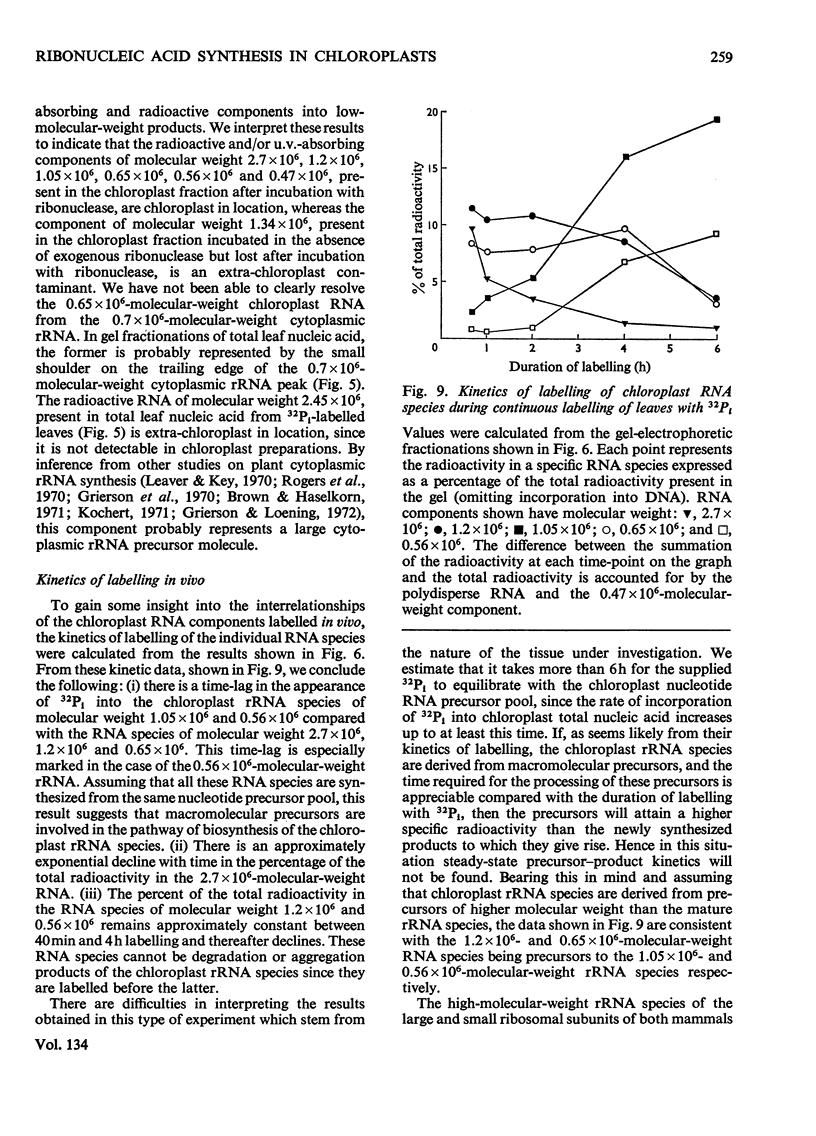
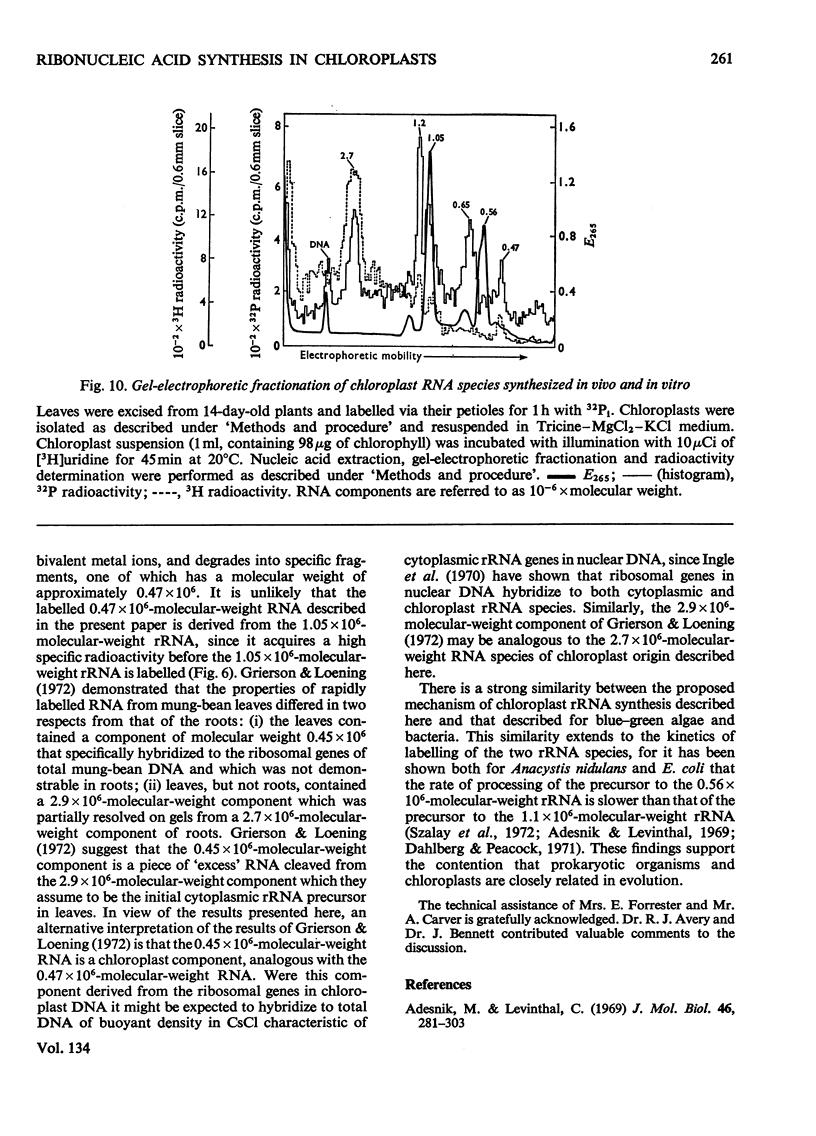
Chloroplasts isolated from young spinach leaves incorporate [3H]uridine into RNA. This incorporation shows an absolute requirement for light and does not occur in lysed chloroplasts. Fractionation by polyacrylamide-gel electrophoresis of the RNA synthesized in vitro reveals a major discrete product of molecular weight 2.7×106 and two minor products of molecular weight 1.2×106 and 0.47×106. These discrete products are super-imposed on a background of polydisperse RNA. The incorporation of 32Pi into chloroplast rRNA species (mol.wt. 1.05×106 and 0.56×106) in excised spinach leaves proceeds after a distinct lag period compared with the incorporation into cytoplasmic rRNA species (mol.wt. 1.34×106 and 0.7×106). Incorporation of 32Pi into chloroplast RNA species of molecular weight 2.7×106, 1.2×106, 0.65×106 and 0.47×106 proceeds without such a time-lag. The kinetics of labelling of the individual RNA components is consistent with the rapidly labelled RNA species of molecular weight 1.2×106 and 0.65×106 being precursors to the more slowly labelled rRNA species of molecular weight 1.05×106 and 0.56×106 respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery R. J., Midgley J. E. Ribosomal ribonucleic acid synthesis in Bacillus subtilis. Biochem J. 1971 Apr;122(2):139–148. doi: 10.1042/bj1220139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. RNA-synthesis in Acetabularia. II. RNA-synthesis in isolated chloroplasts. Protoplasma. 1967;64(1):13–25. doi: 10.1007/BF01257379. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Haselkorn R. Synthesis and maturation of cytoplasmic ribosomal RNA in Euglena gracilis. J Mol Biol. 1971 Aug 14;59(3):491–503. doi: 10.1016/0022-2836(71)90312-3. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Peacock A. C. Studies of 16 and 23 S ribosomal RNA of Escherichia coli using composite gel electrophoresis. J Mol Biol. 1971 Jan 14;55(1):61–74. doi: 10.1016/0022-2836(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Grierson D., Loening U. E. Distinct transcription products of ribosomal genes in two different tissues. Nat New Biol. 1972 Jan 19;235(55):80–82. doi: 10.1038/newbio235080a0. [DOI] [PubMed] [Google Scholar]
- Hecht N. B., Woese C. R. Separation of bacterial ribosomal ribonucleic acid from its macromolecular precursors by polyacrylamide gel electrophoresis. J Bacteriol. 1968 Mar;95(3):986–990. doi: 10.1128/jb.95.3.986-990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Hill R., Walker D. A. Pyocyanine and Phosphorylation with Chloroplasts. Plant Physiol. 1959 May;34(3):240–245. doi: 10.1104/pp.34.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Possingham J. V., Wells R., Leaver C. J., Loening U. E. The properties of chloroplast ribosomal-RNA. Symp Soc Exp Biol. 1970;24:303–325. [PubMed] [Google Scholar]
- Ingle J. Synthesis and Stability of Chloroplast Ribosomal-RNA's. Plant Physiol. 1968 Sep;43(9):1448–1454. doi: 10.1104/pp.43.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochert G. Ribosomal RNA synthesis in Volvox. Arch Biochem Biophys. 1971 Nov;147(1):318–322. doi: 10.1016/0003-9861(71)90339-0. [DOI] [PubMed] [Google Scholar]
- Kossman C. R., Stamato T. D., Pettijohn D. E. Tandem synthesis of the 16S and 23S ribosomal RNA sequences of Escherichia coli. Nat New Biol. 1971 Nov 24;234(47):102–104. doi: 10.1038/newbio234102a0. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Ribosomal RNA synthesis in plants. J Mol Biol. 1970 May 14;49(3):671–680. doi: 10.1016/0022-2836(70)90290-1. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Margulies M. M., Gantt E., Parenti F. In vitro Protein Synthesis by Plastids of Phaseolus vulgaris. II. The Probable Relation Between Ribonuclease Insensitive Amino Acid Incorporation and the Presence of Intact Chloroplasts. Plant Physiol. 1968 Apr;43(4):495–503. doi: 10.1104/pp.43.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Stonington O. G., Kossman C. R. Chain termination of ribosomal RNA synthesis in vitro. Nature. 1970 Oct 17;228(5268):235–239. doi: 10.1038/228235a0. [DOI] [PubMed] [Google Scholar]
- Rogers M. E., Loening U. E., Fraser R. S. Ribosomal RNA precursors in plants. J Mol Biol. 1970 May 14;49(3):681–692. doi: 10.1016/0022-2836(70)90291-3. [DOI] [PubMed] [Google Scholar]
- Scott N. S., Smillie R. M. Evidence for the direction of chloroplasts ribosomal RNA synthesis by chloroplast DNA. Biochem Biophys Res Commun. 1967 Aug 23;28(4):598–603. doi: 10.1016/0006-291x(67)90355-5. [DOI] [PubMed] [Google Scholar]
- Spencer D., Whitfeld P. R. Ribonucleic acid synthesizing activity of spinach chloroplasts and nuclei. Arch Biochem Biophys. 1967 Aug;121(2):336–345. doi: 10.1016/0003-9861(67)90085-9. [DOI] [PubMed] [Google Scholar]
- Stutz E., Noll H. Characterization of cytoplasmic and chloroplast polysomes in plants: evidence for three classes of ribosomal RNA in nature. Proc Natl Acad Sci U S A. 1967 Mar;57(3):774–781. doi: 10.1073/pnas.57.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz E. The kinetic complexity of Euglena gracilis chloroplasts DNA. FEBS Lett. 1970 May 11;8(1):25–28. doi: 10.1016/0014-5793(70)80216-2. [DOI] [PubMed] [Google Scholar]
- Surzycki S. J., Goodenough U. W., Levine R. P., Armstrong J. J. Nuclear and chloroplast control of chloroplast structure and function in Chlamydomonas reinhardi. Symp Soc Exp Biol. 1970;24:13–37. [PubMed] [Google Scholar]
- Szalay A., Munsche D., Wolligiehn R., Parthier B. Ribosomal ribonucleic acid and ribosomal precursor ribonucleic acid in Anacystis nidulans. Biochem J. 1972 Aug;129(1):135–140. doi: 10.1042/bj1290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Function of chloroplast DNA. I. Hybridization studies involving nuclear and chloroplast DNA with RNA from cytoplasmic (80S) and chloroplast (70S) ribosomes. Proc Natl Acad Sci U S A. 1968 Feb;59(2):569–576. doi: 10.1073/pnas.59.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Function of chloroplast DNA. II. Studies on DNA-dependent RNA polymerase activity of tobacco chloroplasts. Biochim Biophys Acta. 1969 Aug 20;186(2):358–372. doi: 10.1016/0005-2787(69)90014-8. [DOI] [PubMed] [Google Scholar]
- Tewari K. K., Wildman S. G. Information content in the chloroplast DNA. Symp Soc Exp Biol. 1970;24:147–179. [PubMed] [Google Scholar]
- Treharne K. J., Stoddart J. L., Pughe J., Paranjothy K., Wareing P. F. Effects of gibberellin and cytokinins on the activity of photosynthetic enzymes and plastid ribosomal RNA synthesis in Phaseolus vulgaris L. Nature. 1970 Oct 10;228(5267):129–131. doi: 10.1038/228129a0. [DOI] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Sager R. Denaturation and the renaturation kinetics of chloroplast DNA from Chlamydomonas reinhardi. J Mol Biol. 1971 Jun 14;58(2):611–622. doi: 10.1016/0022-2836(71)90375-5. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S. Building a bacterial virus. Sci Am. 1967 Jul;217(1):61–passim. [PubMed] [Google Scholar]
- Woodcock C. L., Bogorad L. On the extraction and characterization of ribosomal RNA from Acetabularia. Biochim Biophys Acta. 1970 Dec 14;224(2):639–643. doi: 10.1016/0005-2787(70)90601-5. [DOI] [PubMed] [Google Scholar]


