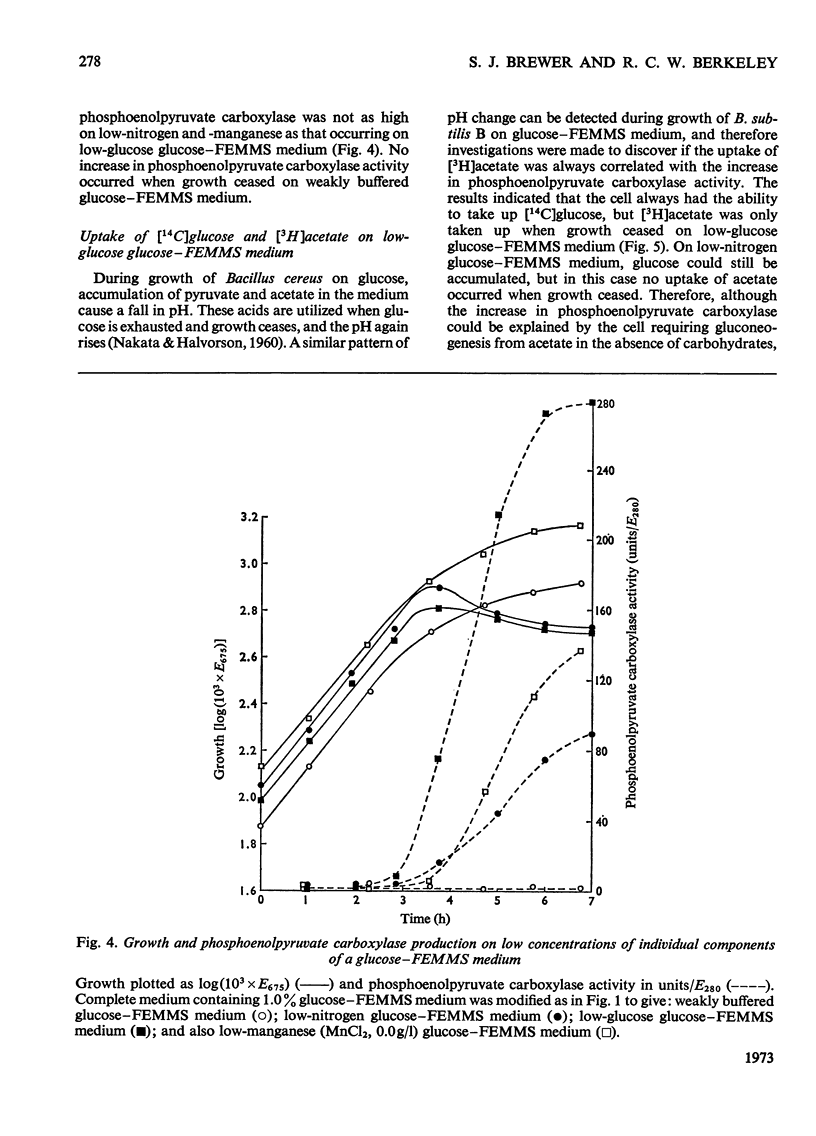
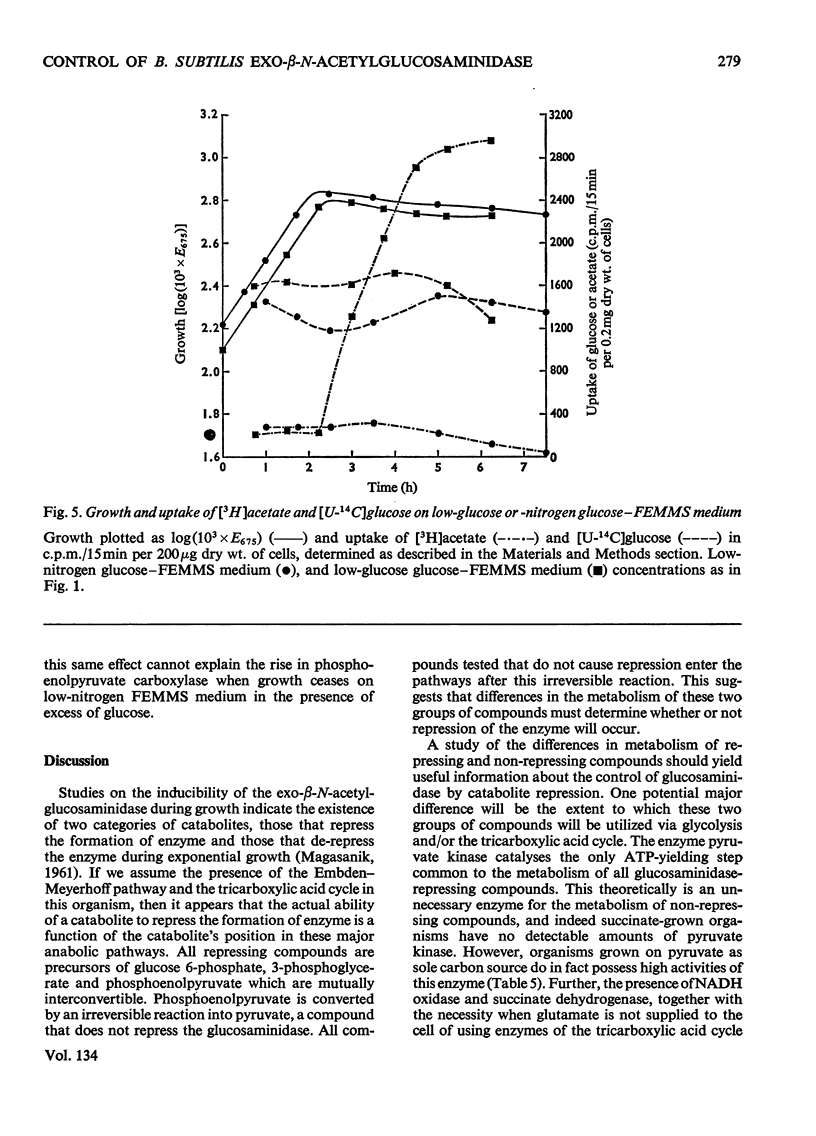
Abstract
1. The control of exo-β-N-acetylglucosaminidase (EC 3.2.1.30) production by Bacillus subtilis B growing on a chemically defined medium was studied. 2. The enzyme was repressed during exponential growth by those carbon sources that enter the glycolytic pathway above the level of phosphoenolpyruvate. When exponential growth ceased as a result of low concentrations of the nitrogen, carbon or metal ion components of the medium, the enzyme was formed and its amount could be increased by the addition of cell-wall fragments as inducer. 3. The enzyme was de-repressed and could be induced during exponential growth on non-glycolytic compounds metabolized directly into pyruvate, acetyl-CoA or tricarboxylic acid cycle intermediates. 4. The major difference in the metabolism of the organism utilizing these two groups of compound was the existence of high activities of phosphoenolpyruvate carboxylase required for gluconeogenesis. 5. It is concluded that the de-repression of glucosaminidase occurs when the only principal change detected in the intermediary metabolism of the organism was the presence of high activities of phosphoenolpyruvate carboxylase. 6. When the organism was grown on media containing repressing compounds, the enzyme was only de-repressed on entry of the cells into the initial stages of sporulation, where phosphoenolpyruvate carboxylase activity, even in the presence of excess of glucose, increased in parallel with glucosaminidase, neutral proteinase and alkaline phosphatase activities. 7. These results suggest a strong link, at the level of the tricarboxylic acid cycle, between the control of phosphoenolpyruvate carboxylase and the control of the de-repression of glucosaminidase and sporulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkeley R. C., Brewer S. J., Ortiz J. M., Gillespie J. B. An exo- -N-acetylglucosaminidase from Bacillus subtilis B; characterization. Biochim Biophys Acta. 1973 May 5;309(1):157–168. doi: 10.1016/0005-2744(73)90327-6. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):263–280. doi: 10.1098/rspb.1967.0065. [DOI] [PubMed] [Google Scholar]
- Elmerich C., Aubert J. P. Role of glutamine synthetase in the repression of bacterial sporulation. Biochem Biophys Res Commun. 1972 Jan 31;46(2):892–897. doi: 10.1016/s0006-291x(72)80225-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J. M., Gillespie J. B., Berkeley R. C. An exo- -N-acetylglucosaminidase from Bacillus subtilis B; extraction and purification. Biochim Biophys Acta. 1972 Nov 10;289(1):174–186. doi: 10.1016/0005-2744(72)90120-9. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Specificity of ction of endo-beta-N-acetylglucosaminidase. Biochem J. 1970 Dec;120(4):735–744. doi: 10.1042/bj1200735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. A., Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as all teric effectors. II. Control of phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Apr 10;244(7):1838–1845. [PubMed] [Google Scholar]


