Abstract
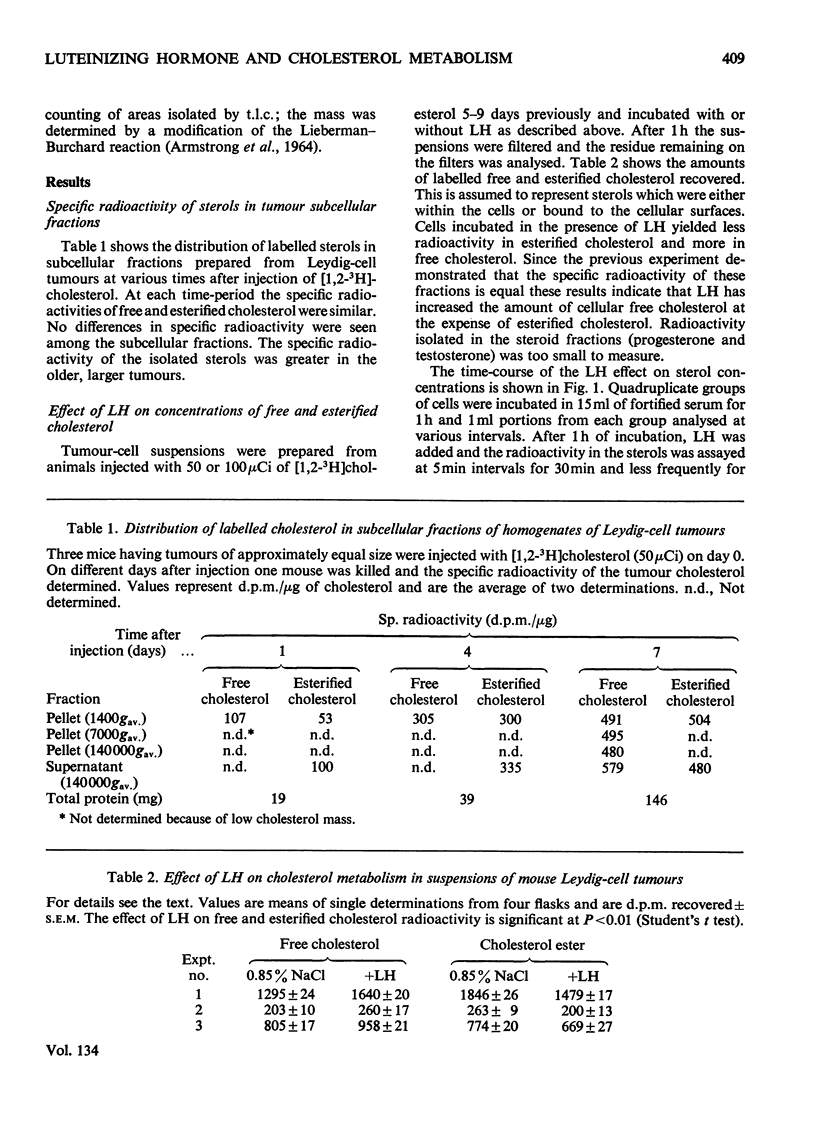
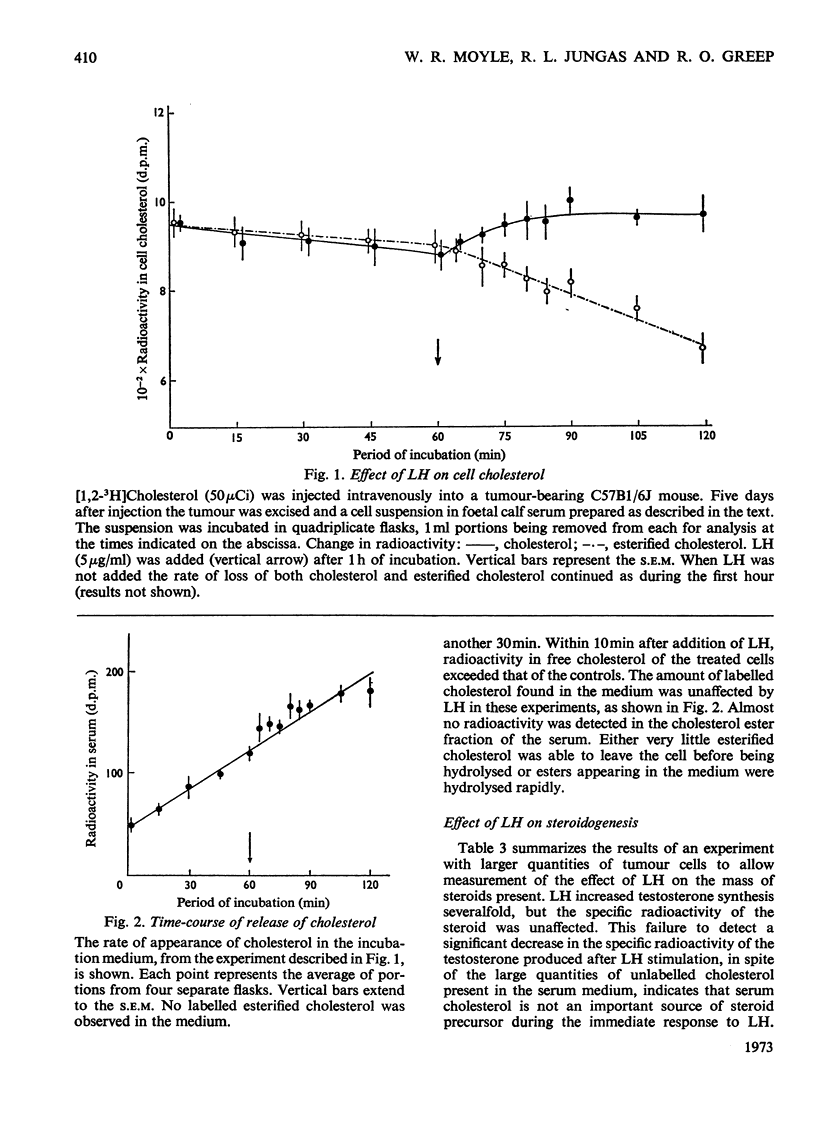
1. Male C57B1/6J mice bearing Leydig-cell tumours known to synthesize steroids in response to luteinizing hormone (LH) were given intravenous injections of [1,2-3H]cholesterol (50–100μCi per animal). Single-cell suspensions were prepared from the tumours 5–9 days after the injection of [3H]cholesterol and were incubated at 37°C in foetal calf serum supplemented with 50mm-Tris–HCl, pH7.4. At various times after the start of incubation cells were collected by filtration of portions of the suspension and their sterols analysed. Within 10min after LH (5μg/ml) or 3′:5′-cyclic AMP (20mm) was added to the cell suspensions an increased conversion of ester cholesterol into free cholesterol could be demonstrated. 2. To observe this rapid effect of LH it was necessary to incubate the cells for 60min before addition of hormone. 3. The specific radioactivity of testosterone produced was approximately equal to that of the intracellular cholesterol regardless of the presence or absence of LH. 4. The amount of free cholesterol produced in response to LH was far greater than that needed for steroid synthesis. 5. Free cholesterol, but not esterified cholesterol, was released into the incubation medium linearly with time and this release was unaffected by LH. LH may stimulate steroidogenesis in part by increasing the concentration of free cholesterol within the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., O'BRIEN J., GREEP R. O. EFFECTS OF LUTEINIZING HORMONE ON PROGESTIN BIOSYNTHESIS IN THE LUTEINIZED RAT OVARY. Endocrinology. 1964 Oct;75:488–500. doi: 10.1210/endo-75-4-488. [DOI] [PubMed] [Google Scholar]
- Armstrong D. T. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent Prog Horm Res. 1968;24:255–319. doi: 10.1016/b978-1-4831-9827-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Armstrong D. T. On the site of action of luteinizing hormone. Nature. 1967 Feb 11;213(5076):633–634. doi: 10.1038/213633a0. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Amstrong D. T. Cholesterol esterase stimulation by luteinizing hormone in luteinized rat ovaries. Endocrinology. 1969 Sep;85(3):474–480. doi: 10.1210/endo-85-3-474. [DOI] [PubMed] [Google Scholar]
- Berhman H. R., Armstrong D. T., Greep R. O. Studies on the rapid cholesterol-depleting and steroidogenic actions of luteinizing hormone in the rat ovary: effects of aminoglutethimide phosphate. Can J Biochem. 1970 Aug;48(8):881–884. doi: 10.1139/o70-138. [DOI] [PubMed] [Google Scholar]
- Cook B., Kaltenbach C. C., Norton H. W., Nalbandov A. V. Synthesis of progesterone in vitro by porcine corpora lutea. Endocrinology. 1967 Sep;81(3):573–584. doi: 10.1210/endo-81-3-573. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M., Walsh D. A., Krebs E. G. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3',5'- monophosphate-stimulated protein kinase. J Biol Chem. 1970 Sep 25;245(18):4849–4851. [PubMed] [Google Scholar]
- EIK-NES K. B. EFFECTS OF GONADOTROPHINS ON SECRETION OF STEROIDS BY THE TESTIS AND OVARY. Physiol Rev. 1964 Oct;44:609–630. doi: 10.1152/physrev.1964.44.4.609. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Young D. G. Site of action of trophic hormones upon the biosynthetic pathways to steroid hormones. Endocrinology. 1968 Mar;82(3):559–568. doi: 10.1210/endo-82-3-559. [DOI] [PubMed] [Google Scholar]
- Herbst A. L. Response of rat ovarian cholesterol to gonadotropins and anterior pituitary hormones. Endocrinology. 1967 Jul;81(1):54–60. doi: 10.1210/endo-81-1-54. [DOI] [PubMed] [Google Scholar]
- Huttunen J. K., Aquino A. A., Steinberg D. A purified triglyceride lipase, lipoprotein in nature, from rat adipose tissue. Biochim Biophys Acta. 1970 Nov 12;224(1):295–298. doi: 10.1016/0005-2787(70)90652-0. [DOI] [PubMed] [Google Scholar]
- Huttunen J. K., Steinberg D., Mayer S. E. ATP-dependent and cyclic AMP-dependent activation of rat adipose tissue lipase by protein kinase from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1970 Sep;67(1):290–295. doi: 10.1073/pnas.67.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. M., Savard K. The stimulation of progesterone synthesis in bovine corpora lutea by adenosine 3',5'-monophosphate. Steroids. 1966 Aug;8(2):133–148. doi: 10.1016/0039-128x(66)90088-2. [DOI] [PubMed] [Google Scholar]
- Marsh J. M. The stimulatory effect of luteinizing hormone on adenyl cyclase in the bovine corpus luteum. J Biol Chem. 1970 Apr 10;245(7):1596–1603. [PubMed] [Google Scholar]
- Moyle W. R., Armstrong D. T. Stimulation of testosterone biosynthesis by luteinizing hormone in transplantable mouse Leydig cell tumors. Steroids. 1970 May;15(5):681–693. doi: 10.1016/s0039-128x(70)80073-3. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Moudgal N. R., Greep R. O. Cessation of steroidogenesis in Leydig cell tumors after removal of luteinizing hormone and adenosine cyclic 3',5'-monophosphate. J Biol Chem. 1971 Aug 25;246(16):4978–4982. [PubMed] [Google Scholar]
- Solod E. A., Armstrong D. T., Greep R. O. Action of luteinizing hormone on conversion of ovarian cholesterol stores to steroids secreted in vivo and synthesized in vitro by the pseudopregnant rabbit ovary. Steroids. 1966 Jun;7(6):607–620. doi: 10.1016/0039-128x(66)90147-4. [DOI] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]


