Abstract
A specific antibody against liver cytosol phosphoenolpyruvate carboxylase (EC 4.1.1.32) was used to isolate the enzyme from liver and adipose tissue. With this technique we have shown that phosphoenolpyruvate carboxylase synthesis in starved rats accounts for 3% of the total synthesis of cytosol protein in each tissue. Re-feeding starved animals decreases this relative rate of phosphoenolpyruvate carboxylase synthesis to 0.2% and 1% respectively in liver and adipose tissue, and the activity of the enzyme in each tissue is decreased to 25% of the starvation value. An additional starvation period is accompanied by an increased rate of enzyme synthesis, but the response to starvation is considerably slower than that caused by re-feeding. The degradation rate of phosphoenolpyruvate carboxylase is also subject to regulation. Thus re-feeding starved animals decreases the half-life of the enzyme in liver from 13h to 5.2h, but the rapid rate of degradation is maintained at least during the first 20h of subsequent starvation. Only slight changes in the degradation rate of phosphoenolpyruvate carboxylase are found in adipose tissue. We conclude that the large alterations in the rate of enzyme synthesis during a starvation–re-feeding cycle are the major cause of fluctuations in activity.
Full text
PDF



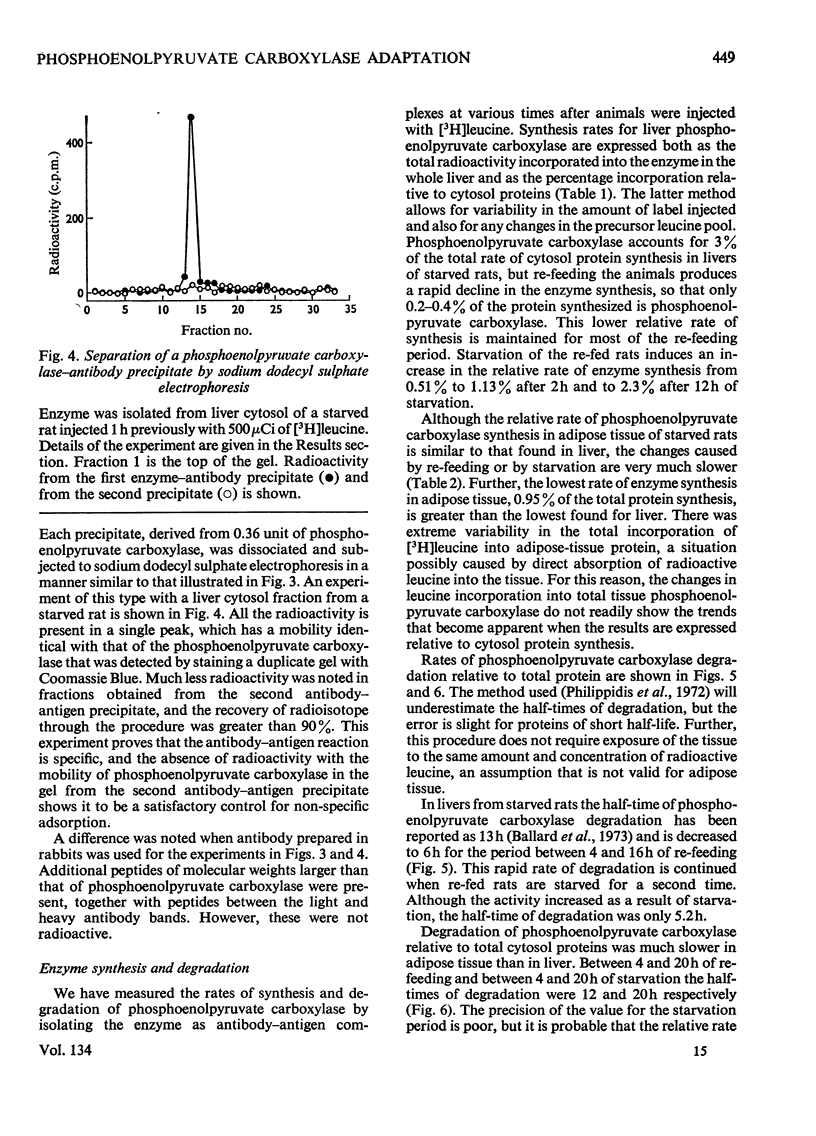
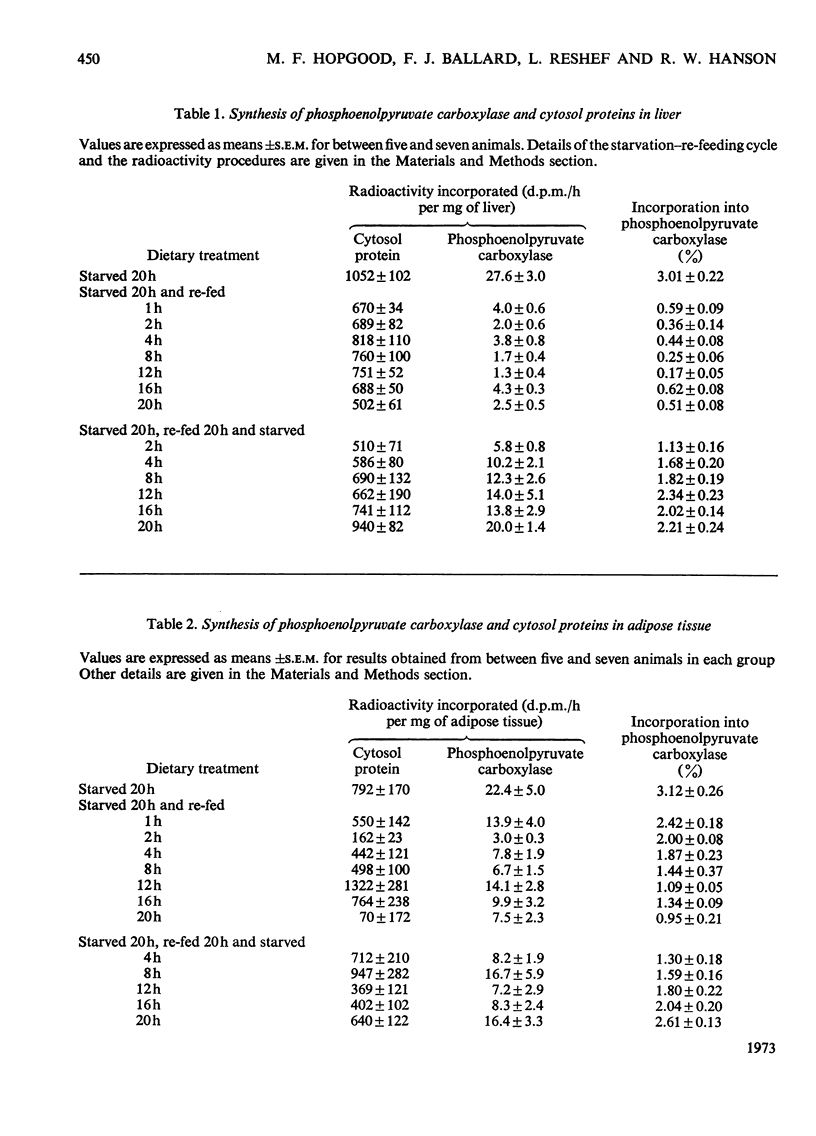



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W., Leveille G. A. Phosphoenolpyruvate carboxykinase and the synthesis of glyceride-glycerol from pyruvate in adipose tissue. J Biol Chem. 1967 Jun 10;242(11):2746–2750. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Ray P. D., Lardy H. A. Studies on the mechanisms underlying adaptive changes in rat liver phosphoenolpyruvate carboxykinase. Biochemistry. 1966 Feb;5(2):555–562. doi: 10.1021/bi00866a022. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Nagai K., Nakagawa H. Cold-adaptation. II. Effect of thyroxine on phosphoenolpyruvate carboxykinase in rat liver in normal and cold environments. J Biochem. 1972 Jan;71(1):125–131. doi: 10.1093/oxfordjournals.jbchem.a129733. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C., Peraino C., Lamar C., Jr, Kennan A. L. Template stability of some enzymes in rat liver and hepatoma. Proc Natl Acad Sci U S A. 1965 Sep;54(3):845–851. doi: 10.1073/pnas.54.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL M., HIATT H. H. THE STABILITY OF LIVER MESSENGER RNA. Proc Natl Acad Sci U S A. 1964 May;51:810–818. doi: 10.1073/pnas.51.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef L., Ballard F. J., Hanson R. W. The role of the adrenals in the regulation of phosphoenolpyruvate carboxykinase of rat adipose tissue. J Biol Chem. 1969 Oct 25;244(20):5577–5581. [PubMed] [Google Scholar]
- Reshef L., Hanson R. W., Ballard F. J. Glyceride-glycerol synthesis from pyruvate. Adaptive changes in phosphoenolpyruvate carboxykinase and pyruvate carboxylase in adipose tissue and liver. J Biol Chem. 1969 Apr 25;244(8):1994–2001. [PubMed] [Google Scholar]
- Reshef L., Meyuhas O., Boshwitz C., Hanson R. W., Ballard F. J. Physiological role and regulation of glyceroneogenesis in rat adipose tissue. Isr J Med Sci. 1972 Mar;8(3):372–381. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- Schrago E., Young J. W., Lardy H. A. Carbohydrate supply as a regulator of rat liver phosphoenolpyruvate carboxykinase activity. Science. 1967 Dec 22;158(3808):1572–1573. doi: 10.1126/science.158.3808.1572. [DOI] [PubMed] [Google Scholar]
- Snoke R. E., Johnston J. B., Lardy H. A. Response of phosphopyruvate carboxylase to tryptophan metabolites and metal ions. Eur J Biochem. 1971 Dec;24(2):342–346. doi: 10.1111/j.1432-1033.1971.tb19692.x. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Martin D. W., Jr Hormones and gene expression. Annu Rev Genet. 1970;4:91–106. doi: 10.1146/annurev.ge.04.120170.000515. [DOI] [PubMed] [Google Scholar]
- Treadow B. R., Khairallah E. A. Regulation of phospho-enol-pyruvate carboxykinase during starvation and glucose repression. Nat New Biol. 1972 Oct 4;239(92):131–133. doi: 10.1038/newbio239131a0. [DOI] [PubMed] [Google Scholar]
- Ward S., Wilson D. L., Gilliam J. J. Methods for fractionation and scintillation counting of radioisotope-labeled polyacrylamide gels. Anal Biochem. 1970 Nov;38(1):90–97. doi: 10.1016/0003-2697(70)90158-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wicks W. D., Lewis W., McKibbin J. B. Induction of phosphoenolpyruvate carboxykinase by N 6 , O 2 '-dibutyryl cyclic AMP in rat liver. Biochim Biophys Acta. 1972 Mar 30;264(1):177–185. doi: 10.1016/0304-4165(72)90129-8. [DOI] [PubMed] [Google Scholar]