Abstract
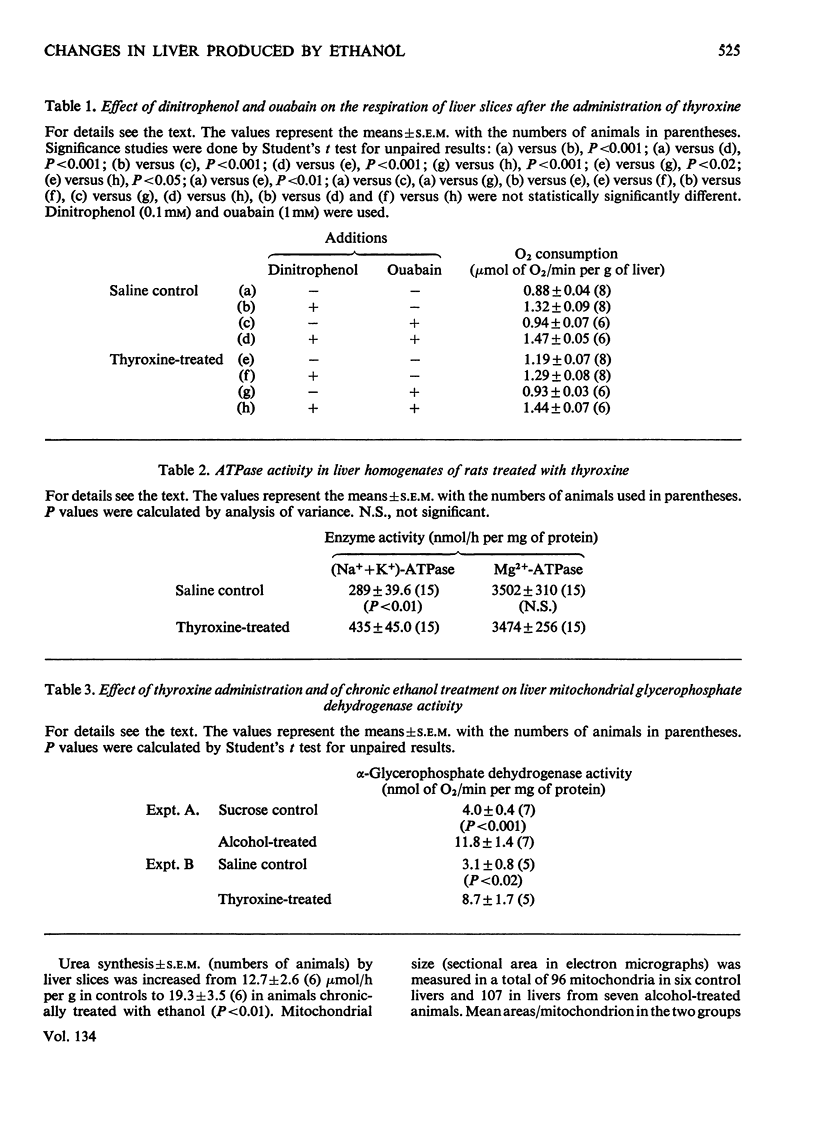
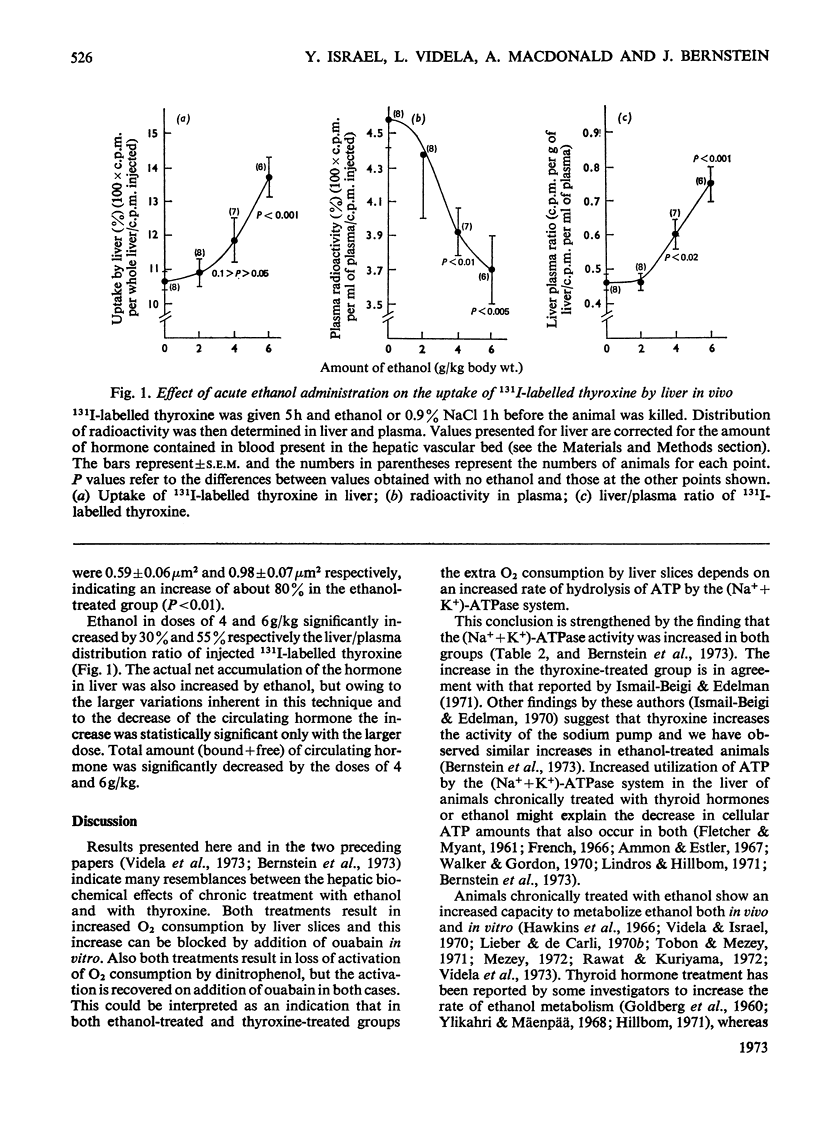
1. Liver slices from rats treated with thyroxine show an increased rate of O2 consumption. The extra consumption, but not the basal respiration, can be abolished by ouabain. 2. Dinitrophenol is not effective in increasing the rate of O2 consumption of liver slices from thyroxine-treated animals but its effectiveness can be recovered in the presence of ouabain. 3. (Na++K+)-stimulated adenosine triphosphatase activity of liver was increased by administration of thyroxine in vivo. No changes were found in total Mg2+-stimulated adenosine triphosphatase activity. 4. Mitochondrial α-glycerophosphate dehydrogenase and microsomal NADPH oxidase activity were increased by both thyroxine and chronic ethanol treatment. 5. Liver slices from animals chronically treated with ethanol synthesize urea at an increased rate. 6. Mitochondrial size (section area) is markedly increased in the liver of animals chronically treated with ethanol. 7. Acute administration of ethanol in doses of 4 and 6g/kg significantly increases the uptake of 131I-labelled thyroxine by the liver. 8. Work reported here, along with results from other investigators, indicates marked similarities between the effects produced in the liver by chronic administration of ethanol and by thyroid hormones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Estler C. J. Influence of acute and chronic administration of alcohol on carbohydrate breakdown and energy metabolism in the liver. Nature. 1967 Oct 14;216(5111):158–159. doi: 10.1038/216158a0. [DOI] [PubMed] [Google Scholar]
- Bernstein J., Videla L., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Changes related to energetic parameters of the cell. Biochem J. 1973 Jun;134(2):515–521. doi: 10.1042/bj1340515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHILSON O. P., SACKS J. Effect of hyperthyroidism on distribution of adenosine phosphates and glycogen in liver. Proc Soc Exp Biol Med. 1959 Jun;101(2):331–332. doi: 10.3181/00379727-101-24928. [DOI] [PubMed] [Google Scholar]
- Carter E. A., Isselbacher K. J. The role of microsomes in the hepatic metabolism of ethanol. Ann N Y Acad Sci. 1971 Jul 6;179:282–294. doi: 10.1111/j.1749-6632.1971.tb46907.x. [DOI] [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- FLETCHER K., MYANT N. B. Partial reversal of the effects of thyroxine on lipid synthesis in rat liver by the addition of cofactors in vitro. J Physiol. 1961 Aug;157:542–564. doi: 10.1113/jphysiol.1961.sp006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S. W. Effect of acute and chronic ethanol ingestion on rat liver ATP. Proc Soc Exp Biol Med. 1966 Mar;121(3):681–685. doi: 10.3181/00379727-121-30860. [DOI] [PubMed] [Google Scholar]
- GOLDBERG M., HEHIR R., HUROWITZ M. Intravenous triiodothyronine in acute alcoholic intoxication. Preliminary report. N Engl J Med. 1960 Dec 29;263:1336–1339. doi: 10.1056/NEJM196012292632603. [DOI] [PubMed] [Google Scholar]
- Gilleland M. J., Shore J. D. Inhibition of horse liver alcohol dehydrogenase by L-3,3',5-triiodothyronine. J Biol Chem. 1969 Oct 10;244(19):5357–5360. [PubMed] [Google Scholar]
- Gillette J. R. Factors affecting drug metabolism. Ann N Y Acad Sci. 1971 Jul 6;179:43–66. doi: 10.1111/j.1749-6632.1971.tb46890.x. [DOI] [PubMed] [Google Scholar]
- Gordon A., Coutsoftides T. The effect of blood pH on the acute distribution of thyroxine in the rat. Endocrinology. 1971 Dec;89(6):1376–1379. doi: 10.1210/endo-89-6-1376. [DOI] [PubMed] [Google Scholar]
- HAGIHARA B. Techniques for the application of polarography to mitochondrial respiration. Biochim Biophys Acta. 1961 Jan 1;46:134–142. doi: 10.1016/0006-3002(61)90656-4. [DOI] [PubMed] [Google Scholar]
- Hassinen I. E., Ylikahri R. H., Kähönen M. T. Regulation of cellular respiration by thyroid hormone. Spectroscopic evidence of mitochondrial control in intact rat liver. Arch Biochem Biophys. 1971 Nov;147(1):255–261. doi: 10.1016/0003-9861(71)90333-x. [DOI] [PubMed] [Google Scholar]
- Hillbom M. E., Pikkarainen P. H. Liver alcohol and sorbitol dehydrogenase activities in hypo- and hyperthyroid rats. Biochem Pharmacol. 1970 Jun;19(6):2097–2103. doi: 10.1016/0006-2952(70)90308-4. [DOI] [PubMed] [Google Scholar]
- Hillbom M. E. Thyroid state and voluntary alcohol consumption of albino rats. Acta Pharmacol Toxicol (Copenh) 1971;29(1):95–105. doi: 10.1111/j.1600-0773.1971.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmail-Beigi F., Edelman I. S. Mechanism of thyroid calorigenesis: role of active sodium transport. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1071–1078. doi: 10.1073/pnas.67.2.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALANT H., SERENY G., CHARLEBOIS R. Evaluation of tri-iodothyronine in the treatment of acute alcoholic intoxication. N Engl J Med. 1962 Jul 5;267:1–6. doi: 10.1056/NEJM196207052670101. [DOI] [PubMed] [Google Scholar]
- KLATSKIN G. The effect of ethyl alcohol on nitrogen excretion in the rat. Yale J Biol Med. 1961 Oct;34:124–143. [PMC free article] [PubMed] [Google Scholar]
- Kiessling K. H. Effect of ethanol on rat liver. VI: A possible correlation between alpha-glycerophosphate oxidase activity and mitochondrial size in male and female rats fed ethanol. Acta Pharmacol Toxicol (Copenh) 1968;26(3):245–252. doi: 10.1111/j.1600-0773.1968.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Kähönen M. T., Ylikahri R. H., Hassinen I. Ethanol metabolism in rats treated with ethyl-alpha-p-chlorophenoxyisobutyrate (clofibrate). Life Sci II. 1971 Jun 22;10(12):661–670. doi: 10.1016/0024-3205(71)90148-2. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Lefevre A. F., DeCarli L. M., Lieber C. S. Effect of ethanol on cholesterol and bile acid metabolism. J Lipid Res. 1972 Jan;13(1):48–55. [PubMed] [Google Scholar]
- Lefèvre A., Adler H., Lieber C. S. Effect of ethanol on ketone metabolism. J Clin Invest. 1970 Oct;49(10):1775–1782. doi: 10.1172/JCI106395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Reduced nicotinamide-adenine dinucleotide phosphate oxidase: activity enhanced by ethanol consumption. Science. 1970 Oct 2;170(3953):78–80. doi: 10.1126/science.170.3953.78. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., Spritz N., DeCarli L. M. Role of dietary, adipose, and endogenously synthesized fatty acids in the pathogenesis of the alcoholic fatty liver. J Clin Invest. 1966 Jan;45(1):51–62. doi: 10.1172/JCI105323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindros K. O., Hillbom M. E. Hepatic redox state and ketone body metabolism during oxidation of ethanol and fructose in normal, hyper- and hypothyroid rats. Ann Med Exp Biol Fenn. 1971;49(3):162–169. [PubMed] [Google Scholar]
- McCarthy K., Lovenberg W., Sjoerdsma A. The mechanism of inhibition of horse liver alcohol dehydrogenase by thyroxine and related compounds. J Biol Chem. 1968 May 25;243(10):2754–2760. [PubMed] [Google Scholar]
- Mendoza D. M., Flock E. V., Owen C. A., Jr, Paris J. Effect of 5,5'-diphenylhydantoin on the metabolism of L-thyroxine-131-I in the rat. Endocrinology. 1966 Jul;79(1):106–118. doi: 10.1210/endo-79-1-106. [DOI] [PubMed] [Google Scholar]
- Mezey E. Duration of the enhanced activity of the microsomal ethanol-oxidizing enzyme system and rate of ethanol degradation in ethanol-fed rats after withdrawal. Biochem Pharmacol. 1972 Jan 15;21(2):137–142. doi: 10.1016/0006-2952(72)90263-8. [DOI] [PubMed] [Google Scholar]
- NEWMAN H., SMITH M. E. Triiodothyronine in acute alcoholic intoxication. Nature. 1959 Mar 7;183(4662):689–690. doi: 10.1038/183689a0. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Surks M. I. Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest. 1968 Jun;47(6):1399–1406. doi: 10.1172/JCI105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGET G. E., THORP J. M. AN EFFECT OF THYROXIN ON THE FINE STRUCTURE OF THE RAT LIVER CELL. Nature. 1963 Sep 28;199:1307–1308. doi: 10.1038/1991307a0. [DOI] [PubMed] [Google Scholar]
- RAWSON R. W. PHYSIOLOGIC EFFECTS OF THYROXINE IN MAN. Mayo Clin Proc. 1964 Aug;39:637–653. [PubMed] [Google Scholar]
- Radomski M. W., Britton A., Schönbaum E. A free fatty acid-free thyroxine relationship in serum. Can J Physiol Pharmacol. 1968 Jan;46(1):119–120. doi: 10.1139/y68-019. [DOI] [PubMed] [Google Scholar]
- Rawat A. K., Kuriyama K. Contribution of "substrate shuttles" in the transport of extramitochondrial reducing equivalents by hepatic mitochondria from chronic alcohol-fed mice. Arch Biochem Biophys. 1972 Sep;152(1):44–52. doi: 10.1016/0003-9861(72)90191-9. [DOI] [PubMed] [Google Scholar]
- Rawat A. K., Lundquist F. Influence of thyroxine on the metabolism of ethanol and glycerol in rat liver slices. Eur J Biochem. 1968 Jun;5(1):13–17. doi: 10.1111/j.1432-1033.1968.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Rodrigo C., Antezana C., Baraona E. Fat and nitrogen balances in rats with alcohol-induced fatty liver. J Nutr. 1971 Oct;101(10):1307–1310. doi: 10.1093/jn/101.10.1307. [DOI] [PubMed] [Google Scholar]
- Rubin E., Beattie D. S., Toth A., Lieber C. S. Structural and functional effects of ethanol on hepatic mitochondria. Fed Proc. 1972 Jan-Feb;31(1):131–140. [PubMed] [Google Scholar]
- Ruegamer W. R., Newman G. H., Richert D. A., Westerfeld W. W. Specificity of the alpha-glycerophosphate dehydrogenase and malic enzyme response to thyroxine. Endocrinology. 1965 Oct;77(4):707–715. doi: 10.1210/endo-77-4-707. [DOI] [PubMed] [Google Scholar]
- Ruegamer W. R., Ryan N. T., Richert D. A., Westerfeld W. W. The effects of p-chlorophenoxyisobutyrate on the turnover rate and distribution of thyroid hormone in the rat. Biochem Pharmacol. 1969 Mar;18(3):613–624. doi: 10.1016/0006-2952(69)90086-0. [DOI] [PubMed] [Google Scholar]
- SMITH D. E., FALLIS N. E., TETREAULT L. Effect of triiodothyronine on rate of alcohol oxidation in dogs. N Engl J Med. 1963 Jan 10;268:91–92. doi: 10.1056/NEJM196301102680210. [DOI] [PubMed] [Google Scholar]
- Shamoto M. Age differences in the ultrastructure of hepatic cells of thyroxine-treated rats. J Gerontol. 1968 Jan;23(1):1–8. doi: 10.1093/geronj/23.1.1. [DOI] [PubMed] [Google Scholar]
- TAPLEY D. F. MODE AND SITE OF ACTION OF THYROXINE. Mayo Clin Proc. 1964 Aug;39:626–636. [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O. Control of basal metabolic rate by thyroid hormones and cellular function. Nature. 1962 Mar 17;193:1058–1060. doi: 10.1038/1931058a0. [DOI] [PubMed] [Google Scholar]
- Tobon F., Mezey E. Effect of ethanol administration on hepatic ethanol and drug-metabolizing enzymes and on rates of ethanol degradation. J Lab Clin Med. 1971 Jan;77(1):110–121. [PubMed] [Google Scholar]
- Videla L., Bernstein J., Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973 Jun;134(2):507–514. doi: 10.1042/bj1340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla L., Israel Y. Factors that modify the metabolism of ethanol in rat liver and adaptive changes produced by its chronic administration. Biochem J. 1970 Jun;118(2):275–281. doi: 10.1042/bj1180275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Gordon E. R. Biochemical aspects associated with an ethanol-induced fatty liver. Biochem J. 1970 Sep;119(3):511–516. doi: 10.1042/bj1190511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfeld W. W., Richert D. A., Ruegamer W. R. The role of the thyroid hormone in the effect of p-chlorophenoxyisobutyrate in rats. Biochem Pharmacol. 1968 Jun;17(6):1003–1016. doi: 10.1016/0006-2952(68)90359-6. [DOI] [PubMed] [Google Scholar]
- Ylikahri R. H., Mäenpä P. H. Rate of ethanol metabolism in fed and starved rats after thyroxine treatment. Acta Chem Scand. 1968;22(5):1707–1709. doi: 10.3891/acta.chem.scand.22-1707. [DOI] [PubMed] [Google Scholar]
- Young J. W. Effects of D- and L-thyroxine on enzymes in liver and adipose tissue of rats. Am J Physiol. 1968 Feb;214(2):378–383. doi: 10.1152/ajplegacy.1968.214.2.378. [DOI] [PubMed] [Google Scholar]


