Abstract
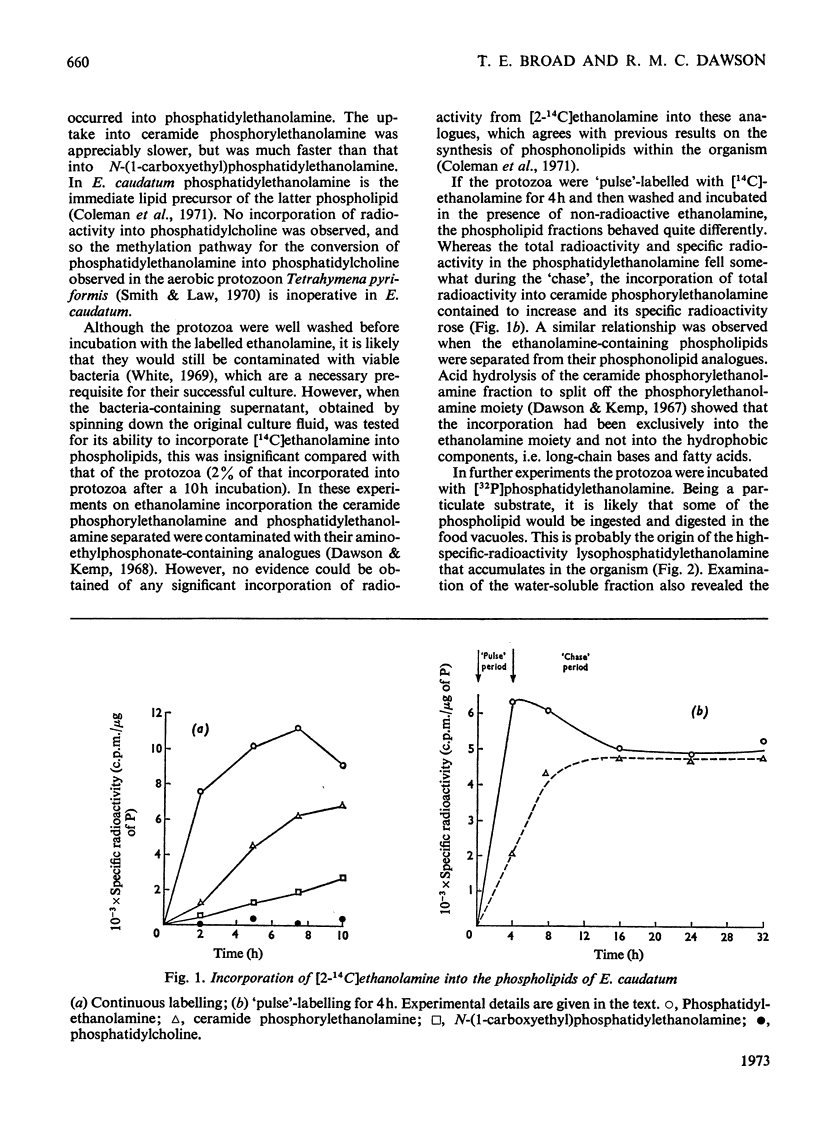
From `pulse'-labelling experiments of Entodinium caudatum with [14C]ethanolamine and by incubating the organism with [32P]phosphatidylethanolamine it is concluded that phosphatidylethanolamine can act as a direct precursor of the phosphorylethanolamine moiety of ceramide phosphorylethanolamine. The phosphorylethanolamine is probably never liberated in the free form but is transferred directly to a ceramide or ceramide-containing acceptor. The results are also in agreement with previous conclusions that phosphatidylethanolamine is the direct lipid precursor of N-(1-carboxyethyl)phosphatidylethanolamine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLEMAN G. S. The preparation and survival of almost bacteria-free suspensions of Entodinium caudatum. J Gen Microbiol. 1962 Jun;28:271–281. doi: 10.1099/00221287-28-2-271. [DOI] [PubMed] [Google Scholar]
- Coleman G. S., Kemp P., Dawson R. M. The catabolism of phosphatidylethanolamine by the rumen protozoon Entodinium caudatum and its conversion into the N-(1-carboxyethyl) derivative. Biochem J. 1971 Jun;123(1):97–104. doi: 10.1042/bj1230097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., BANGHAM A. D. The activation of surface films of lecithin by amphipathic molecules. Biochem J. 1959 Jul;72:493–496. doi: 10.1042/bj0720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Kemp P. Isolation of ceramide phosphorylethanolamine from the blowfly Calliphora erythrocephala. Biochem J. 1968 Jan;106(1):319–320. doi: 10.1042/bj1060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Kemp P. The aminoethylphosphonate-containing lipids of rumen protozoa. Biochem J. 1967 Nov;105(2):837–842. doi: 10.1042/bj1050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Kemp P. The effect of defaunation on the phospholipids and on the hydrogenation of unsaturated fatty acids in the rumen. Biochem J. 1969 Nov;115(2):351–352. doi: 10.1042/bj1150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Marggraf W. D., Koch M. A., Anderer F. A. Evidence for a new biosynthetic pathway of sphingomyelin in SV 40 transformed mouse cells. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1345–1352. doi: 10.1016/0006-291x(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Hildenbrandt G. R., Abraham T., Bieber L. L. Metabolism of ceramide phosphorylethanolamine, phosphatidylinositol, phosphatidylserine and phosphatidylglycerol by housefly larvae. Lipids. 1971 Jul;6(7):508–516. doi: 10.1007/BF02531237. [DOI] [PubMed] [Google Scholar]
- Hori T., Sugita M., Arakawa I. Structural elucidation of sphingoethanolamine and its distribution in aquatic animals. Biochim Biophys Acta. 1968 Jan 10;152(1):211–213. [PubMed] [Google Scholar]
- LaBach J. P., White D. C. Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res. 1969 Sep;10(5):528–534. [PubMed] [Google Scholar]
- Smith J. D., Law J. H. Phosphatidylcholine biosynthesis in Tetrahymena pyriformis. Biochim Biophys Acta. 1970 Feb 10;202(1):141–152. doi: 10.1016/0005-2760(70)90225-0. [DOI] [PubMed] [Google Scholar]
- White R. W. Viable bacteria inside the rumen ciliate Entodinium caudatum. J Gen Microbiol. 1969 Jun;56(3):403–408. doi: 10.1099/00221287-56-3-403. [DOI] [PubMed] [Google Scholar]


