Abstract
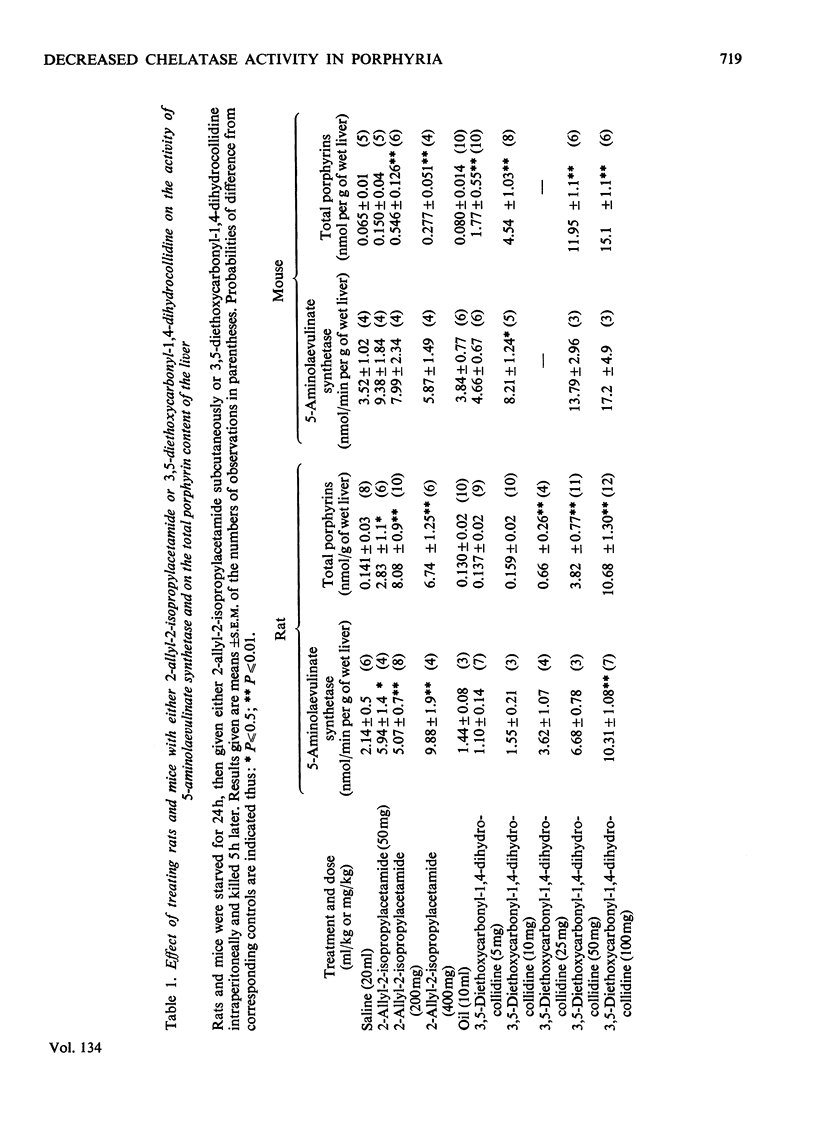
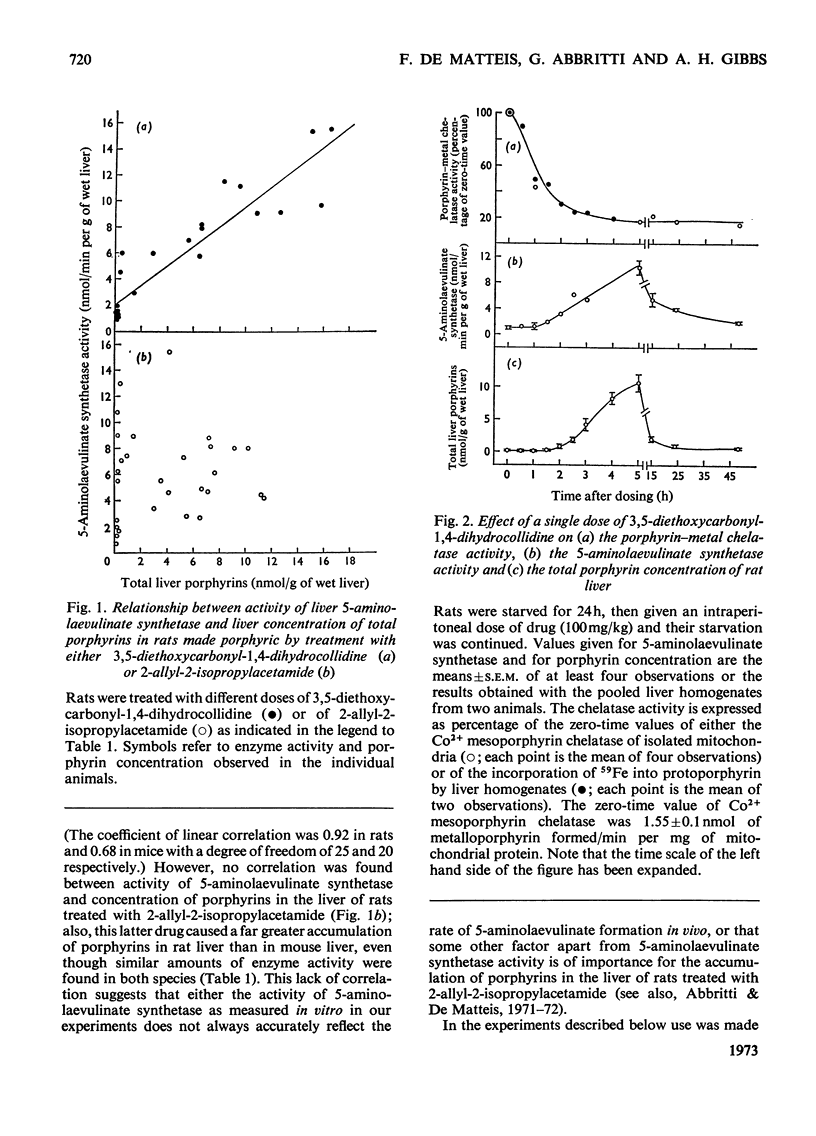
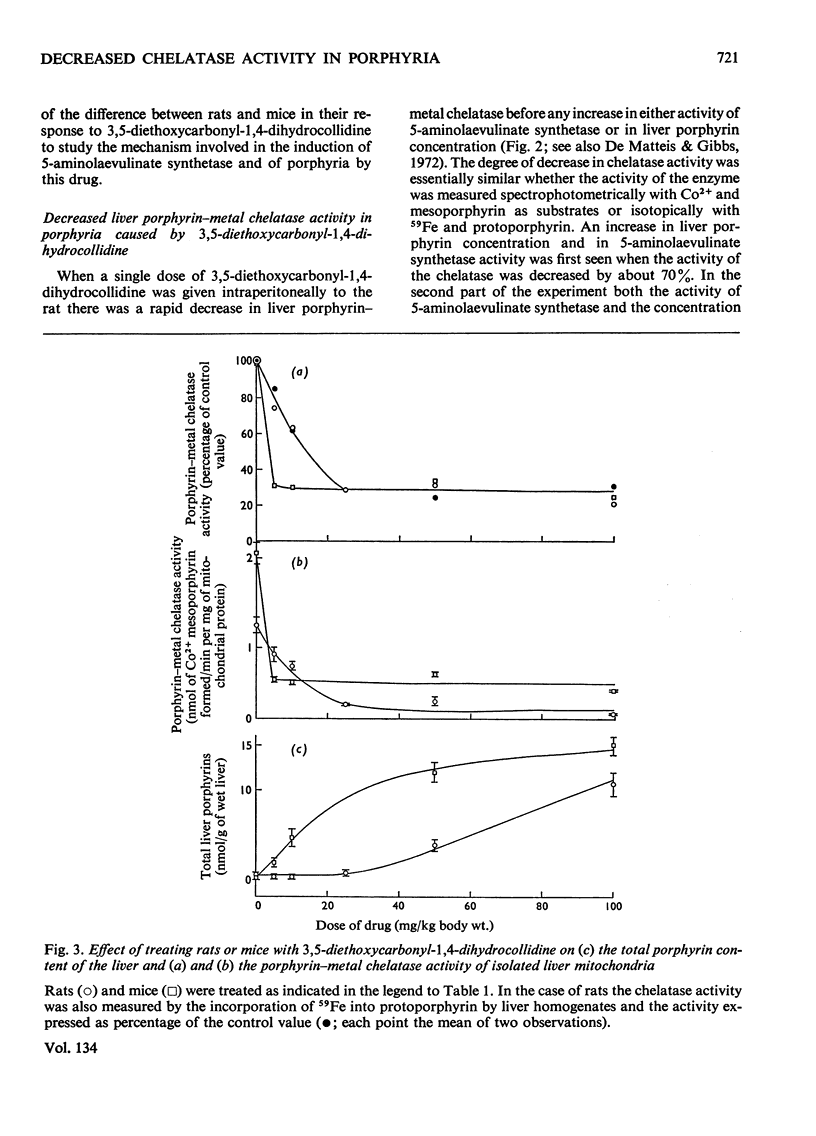
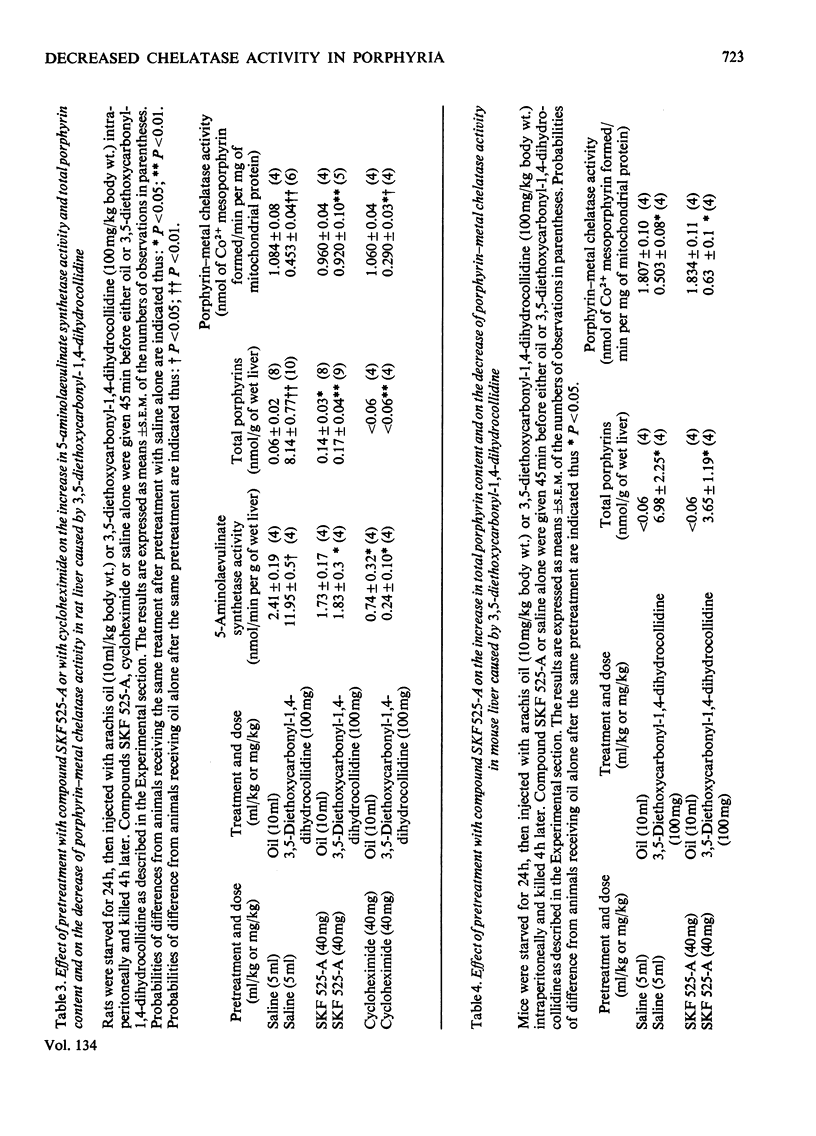
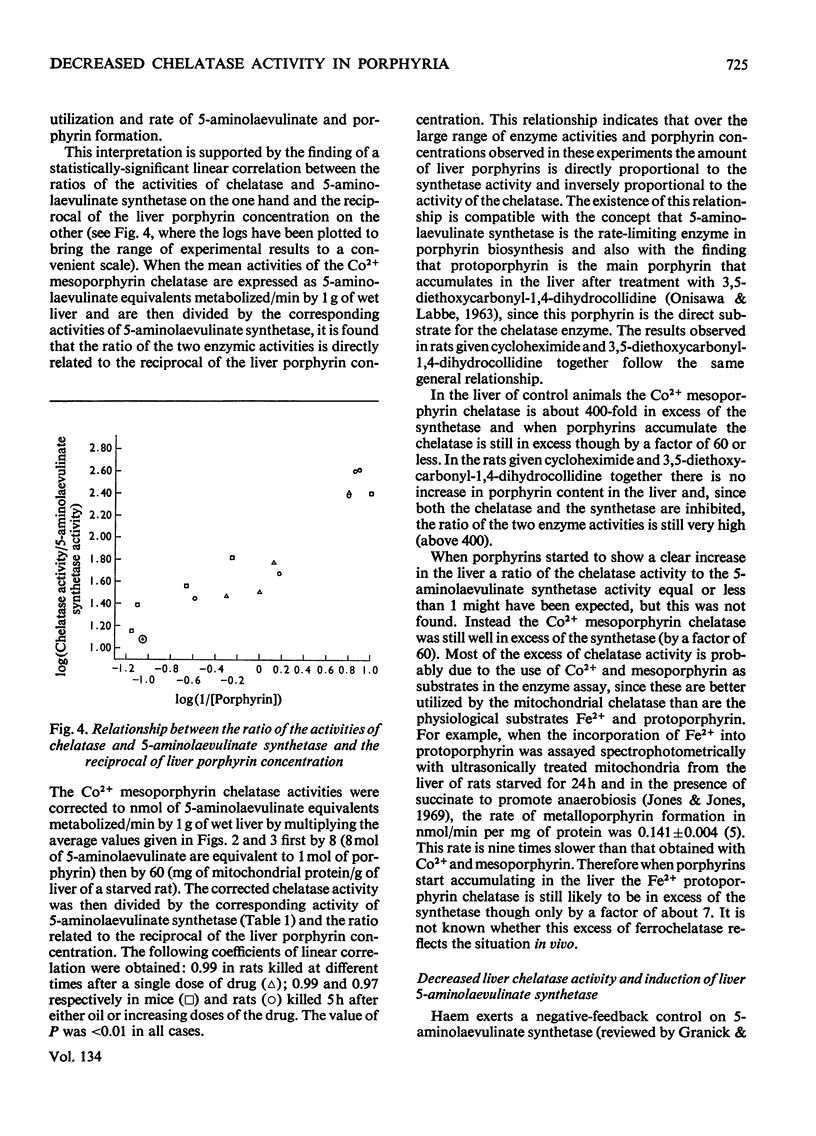
1. A difference has been found between rats and mice in their sensitivity to the porphyrogenic effect of drugs. Mice are more sensitive than rats to 3,5-diethoxycarbonyl-1,4-dihydrocollidine, but less sensitive than rats to 2-allyl-2-isopropylacetamide. 2. Use has been made of this difference in sensitivity to ascertain the importance of the decrease of liver porphyrin–metal chelatase activity in porphyria caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Mice, which are more sensitive than rats to the stimulation of 5-aminolaevulinate caused by this drug, are also more sensitive with respect to the decrease of chelatase activity. 3. In both species, after treatment with 3,5-diethoxycarbonyl-1,4-dihydrocollidine, the ratio between chelatase activity and 5-aminolaevulinate activity is linear with respect to the reciprocal of the liver porphyrin concentration. This suggests that under these conditions the degree of porphyrin accumulation depends on the balance between rate of porphyrin formation and rate of porphyrin utilization. 4. Compound SKF 525-A (2-diethylaminoethyl 3,3-diphenylpropylacetate) when given before 3,5-diethoxycarbonyl-1,4-dihydrocollidine prevents the appearance of porphyria in the rat and also largely prevents the decrease of chelatase activity. In the mouse it is much less effective in preventing porphyria and it is almost completely inactive in protecting the chelatase from a decrease in activity. 5. Cycloheximide, when given before 3,5-diethoxycarbonyl-1,4-dihydrocollidine also inhibits the induction of 5-aminolaevulinate synthetase and the appearance of porphyria in the rat, but does not prevent the decrease of chelatase activity. These results suggest that two successive stages can be distinguished in the induction process: a first stage leading to inhibition of haem synthesis and a second stage requiring synthesis of protein in the liver and leading to stimulation of 5-aminolaevulinate synthetase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Adenosine triphosphatase in the microsomal fraction from rat brain. Biochem J. 1962 Jun;83:527–533. doi: 10.1042/bj0830527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbritti G., De Matteis F. Decreased levels of cytochrome P-450 and catalase in hepatic porphyria caused by substituted acetamides and barbiturates. Importance of the allyl group in the molecule of the active drugs. Chem Biol Interact. 1972 Mar;4(4):281–286. doi: 10.1016/0009-2797(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Creighton J. M., Racz W. J., Tyrrell D. L., Schneck D. W., Marks G. S. Experimental porphyria. II. Drug-induced porphyrin biosynthesis. S Afr Med J. 1971 Sep 25;:79–81. [PubMed] [Google Scholar]
- DE MATTEIS F., RIMINGTON C. Disturbance of porphyrin metabolism caused by griseofulvin in mice. Br J Dermatol. 1963 Mar;75:91–104. doi: 10.1111/j.1365-2133.1963.tb13945.x. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. Stimulation of liver 5-aminolaevulinate synthetase by drugs and its relevance to drug-induced accumulation of cytochrome P-450. Studies with phenylbutazone and 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Biochem J. 1972 Mar;126(5):1149–1160. doi: 10.1042/bj1261149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- Gross S. R., Hutton J. J. Induction of hepatic delta-aminolevulinic acid synthetase activity in strains of inbred mice. J Biol Chem. 1971 Feb 10;246(3):606–614. [PubMed] [Google Scholar]
- Hutton J. J., Gross S. R. Chemical induction of hepatic porphyria in inbred strains of mice. Arch Biochem Biophys. 1970 Nov;141(1):284–292. doi: 10.1016/0003-9861(70)90134-7. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- Levin W., Jacobson M., Kuntzman R. Incorporation of radioactive- -aminolevulinic acid into microsomal cytochrome P 450 : selective breakdown of the hemoprotein by allylisopropylacetamide and carbon tetrachloride. Arch Biochem Biophys. 1972 Jan;148(1):262–269. doi: 10.1016/0003-9861(72)90140-3. [DOI] [PubMed] [Google Scholar]
- Lin C., Chang R., Magat J., Symchowicz S. Metabolism of ( 14 C)griseofulvin in the mouse. J Pharm Pharmacol. 1972 Nov;24(11):911–913. doi: 10.1111/j.2042-7158.1972.tb08915.x. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Marver H. S. Chemically induced porphyria: increased microsomal heme turnover after treatment with allylisopropylacetamide. Science. 1971 Jan 8;171(3966):64–66. doi: 10.1126/science.171.3966.64. [DOI] [PubMed] [Google Scholar]
- ONISAWA J., LABBE R. F. Effects of diethyl-1, 4-dihydro-2, 4,6-trimethylpyridine-3,5-dicarboxylate on the metabolism of porphyrins and iron. J Biol Chem. 1963 Feb;238:724–727. [PubMed] [Google Scholar]
- Racz W. J., Marks G. S. Drug-induced porphyrin biosynthesis. IV. Investigation of the differences in response of isolated liver cells and the liver of the intact chick embryo to porphyria-inducing drugs. Biochem Pharmacol. 1972 Jan 15;21(2):143–151. doi: 10.1016/0006-2952(72)90264-x. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Manning J., Marver H. S. The induction of -aminolevulinic acid synthetase in cultured liver cells. The effects of end product and inhibitors of heme synthesis. J Biol Chem. 1972 May 10;247(9):2820–2827. [PubMed] [Google Scholar]
- Symchowicz S., Wong K. K. Metabolism of griseofulvin-14C; studies in vivo. Biochem Pharmacol. 1966 Oct;15(10):1595–1600. doi: 10.1016/0006-2952(66)90203-6. [DOI] [PubMed] [Google Scholar]
- Tephly T. R., Hasegawa E., Baron J. Effect of drugs on heme synthesis in the liver. Metabolism. 1971 Feb;20(2):200–214. doi: 10.1016/0026-0495(71)90092-8. [DOI] [PubMed] [Google Scholar]


