Abstract
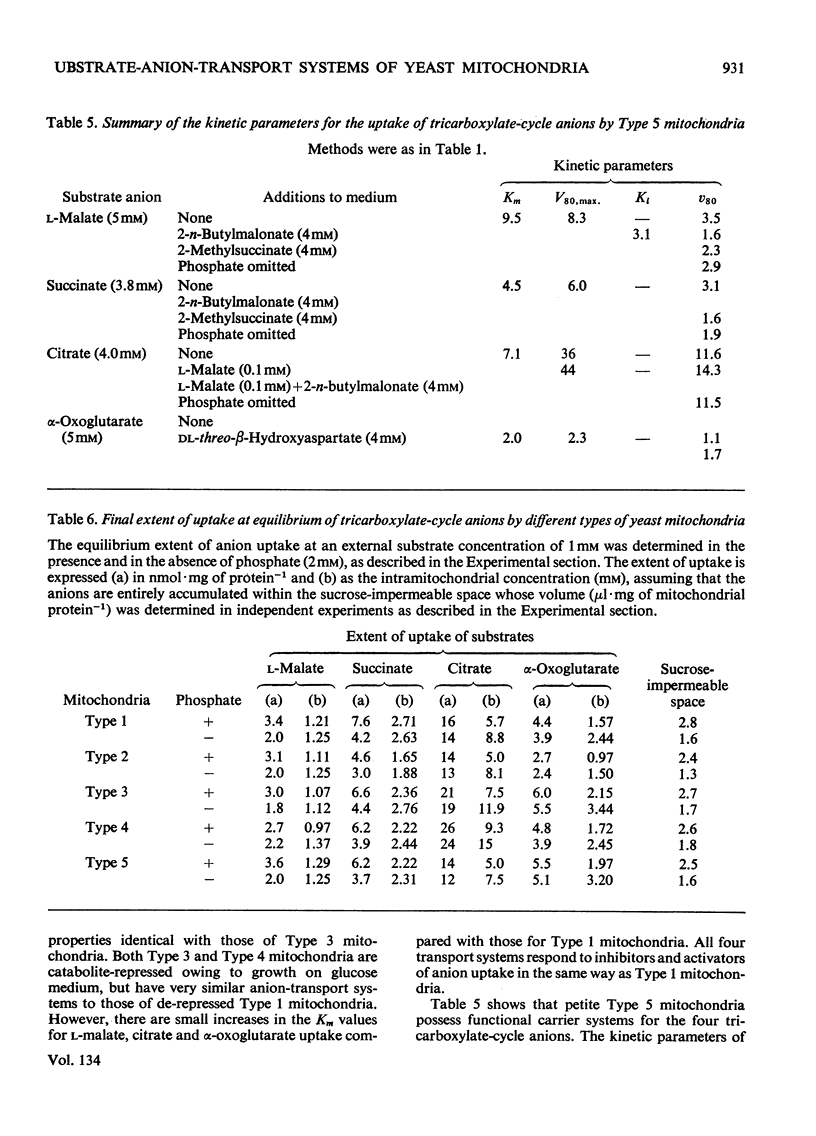
1. Kinetic and equilibrium parameters for the uptake of l-malate, succinate, citrate and α-oxoglutarate by fully functional mitochondria of Saccharomyces cerevisiae were determined. 2. The uptake of l-malate and succinate is mediated by a common carrier, and two other distinct carriers mediate the uptake of citrate and α-oxoglutarate. 3. The properties of the carrier systems for l-malate, succinate and citrate closely resemble those of mammalian mitochondria, but the α-oxoglutarate carrier differs from the mammalian system in minor respects. 4. The composition of the yeast mitochondria was extensively manipulated by (a) anaerobiosis, (b) catabolite repression, (c) inhibition of mitochondrial protein synthesis and (d) elimination of mitochondrial DNA by mutation. 5. The carrier systems for l-malate, succinate, citrate and α-oxoglutarate are essentially similar in the five different types of mitochondria. 6. It is concluded that all the protein components of the carrier systems for l-malate, succinate, citrate and α-oxoglutarate are coded by nuclear genes and synthesized extramitochondrially by cell-sap ribosomes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E., BARTLEY W. The permeability of isolated rat-liver mitochondria to sucrose, sodium chloride and potassium chloride at 0 degrees. Biochem J. 1958 Jun;69(2):223–236. doi: 10.1042/bj0690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Chavin S. I. Isolation and study of functional membrane proteins Present status and future prospects. FEBS Lett. 1971 May 20;14(5):269–282. doi: 10.1016/0014-5793(71)80278-8. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey P. J., Yu R., Linnane A. W. The intracellular site of formation of the mitochondrial protein synthetic system. Biochem Biophys Res Commun. 1969 Jul 7;36(1):30–34. doi: 10.1016/0006-291x(69)90644-5. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Krebs H. A. The permeability of mitochondria to oxaloacetate and malate. Biochem J. 1968 May;107(5):659–667. doi: 10.1042/bj1070659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam J. M., Perkins M., Linnane A. W. Biogenesis of mitochondria. A requirement for mitochondrial protein synthesis for the formation of a normal adenine nucleotide transporter in yeast mitochondria. Biochem J. 1973 Aug;134(4):935–947. doi: 10.1042/bj1340935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Trembath M. K., Linnane A. W., Lukins H. B. Biogenesis of mitochondria. 30. An analysis of polarity of mitochondrial gene recombination and transmission. Mol Gen Genet. 1973 Mar 27;122(1):37–51. doi: 10.1007/BF00337972. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Kolarov J., Subík J., Kovac L. Oxidative phosphorylation in yeast. 8. Osmotic and permeability properties of mitochondria isolated from wild-type yeast and from a respiration-deficient mutant. Biochim Biophys Acta. 1972 Jun 23;267(3):457–464. doi: 10.1016/0005-2728(72)90173-9. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Lukins H. B., Jollow D., Wallace P. G., Linnane A. W. The biogenesis of mitochondria. 7. The effects of glucose repression on the lipid and enzyme composition of mitochondria from yeast. Aust J Exp Biol Med Sci. 1968 Dec;46(6):651–665. [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Mitochondrial DNA deficient petite mutants of yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):989–996. doi: 10.1016/0006-291x(70)90422-5. [DOI] [PubMed] [Google Scholar]
- Perkins M., Haslam J. M., Linnane A. W. The effects of physiological and genetic manipulation on the anion transport systems of yeast mitochondria. FEBS Lett. 1972 Sep 15;25(2):271–274. doi: 10.1016/0014-5793(72)80501-5. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Formation of yeast mitochondria. 3. Biochemical properties of mitochondria isolated from a cytoplasmic petite mutant. J Bioenerg. 1970 Jul;1(2):113–138. doi: 10.1007/BF01515977. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Wallace P. G., Huang M., Linnane A. W. The biogenesis of mitochondria. II. The influence of medium composition on the cytology of anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1968 May;37(2):207–220. doi: 10.1083/jcb.37.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 13. The isolation of mitochondrial structures from anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1970 Jul;46(1):88–96. doi: 10.1083/jcb.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger E. A distinct class of ribosomal RNA components in yeast mitochondria as revealed by gradient centrifugation and by DNA-RNA-hybridization. Hoppe Seylers Z Physiol Chem. 1967 Dec;348(12):1701–1704. [PubMed] [Google Scholar]
- van Dam K., Tsou C. S. Accumulation of substrates by mitochondria. Biochim Biophys Acta. 1968 Oct 1;162(3):301–309. doi: 10.1016/0005-2728(68)90116-3. [DOI] [PubMed] [Google Scholar]



