Abstract
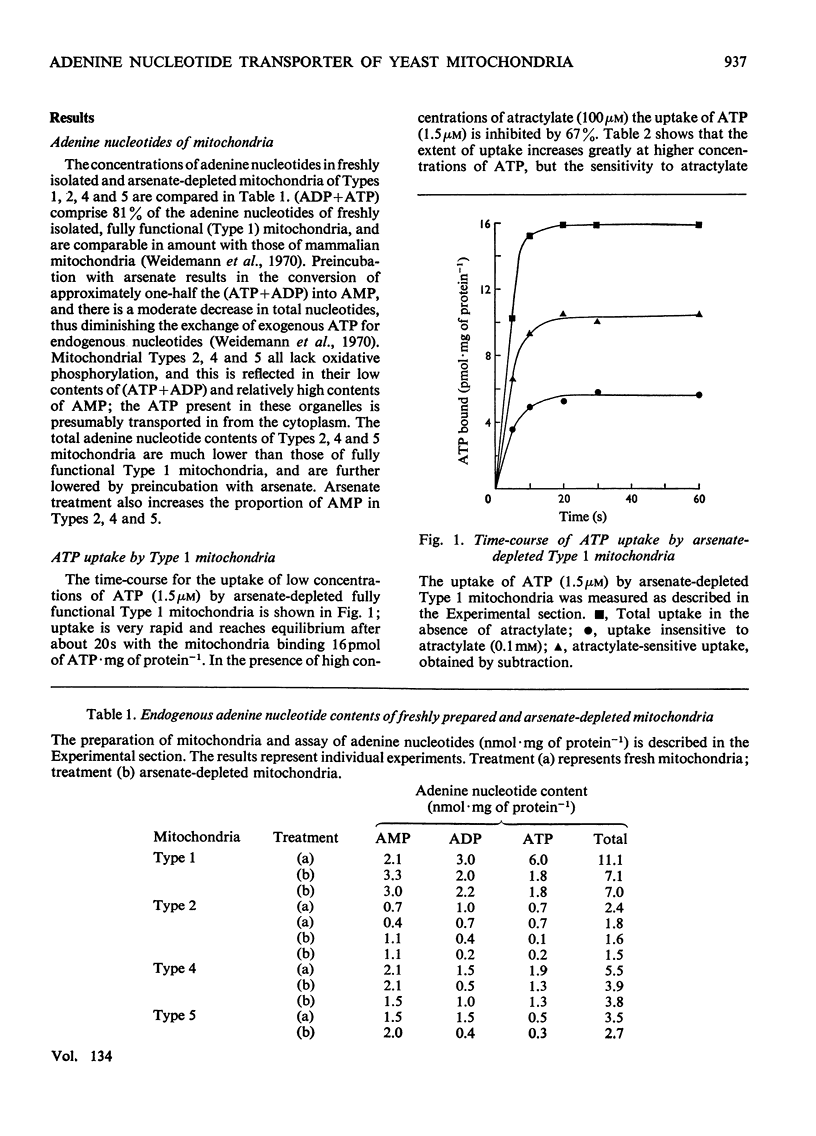
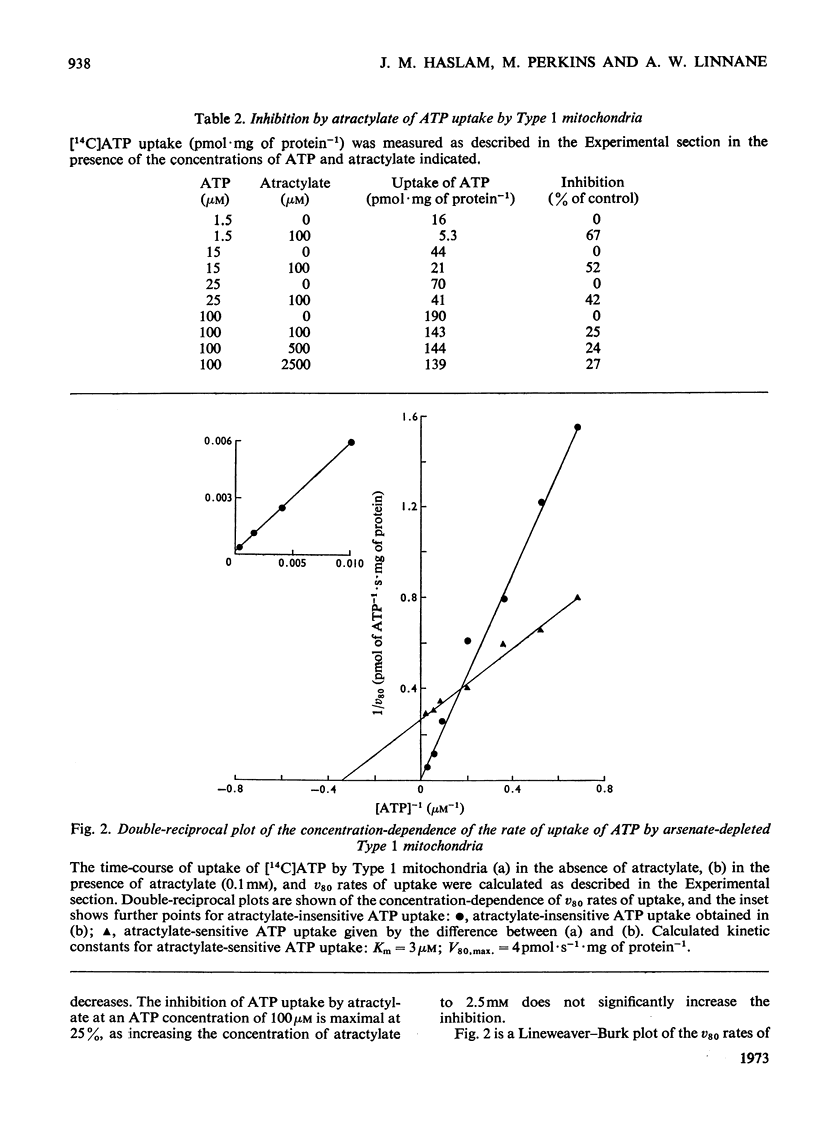
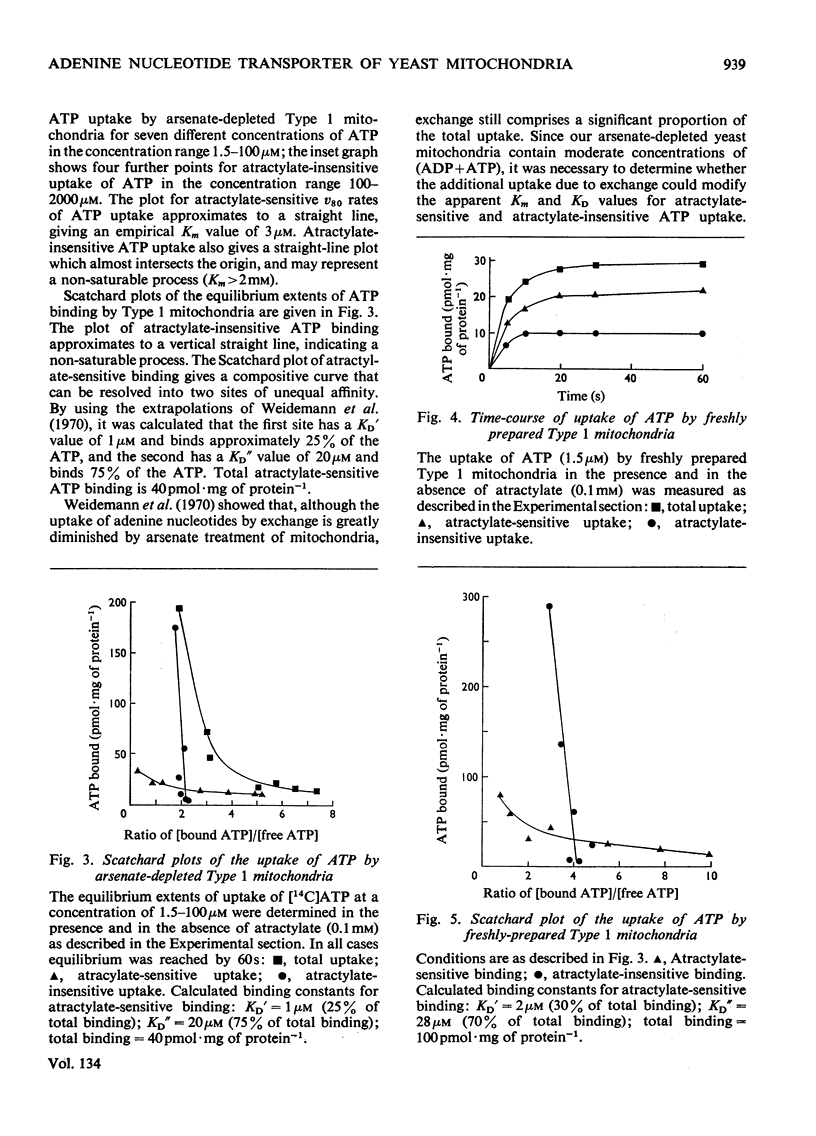
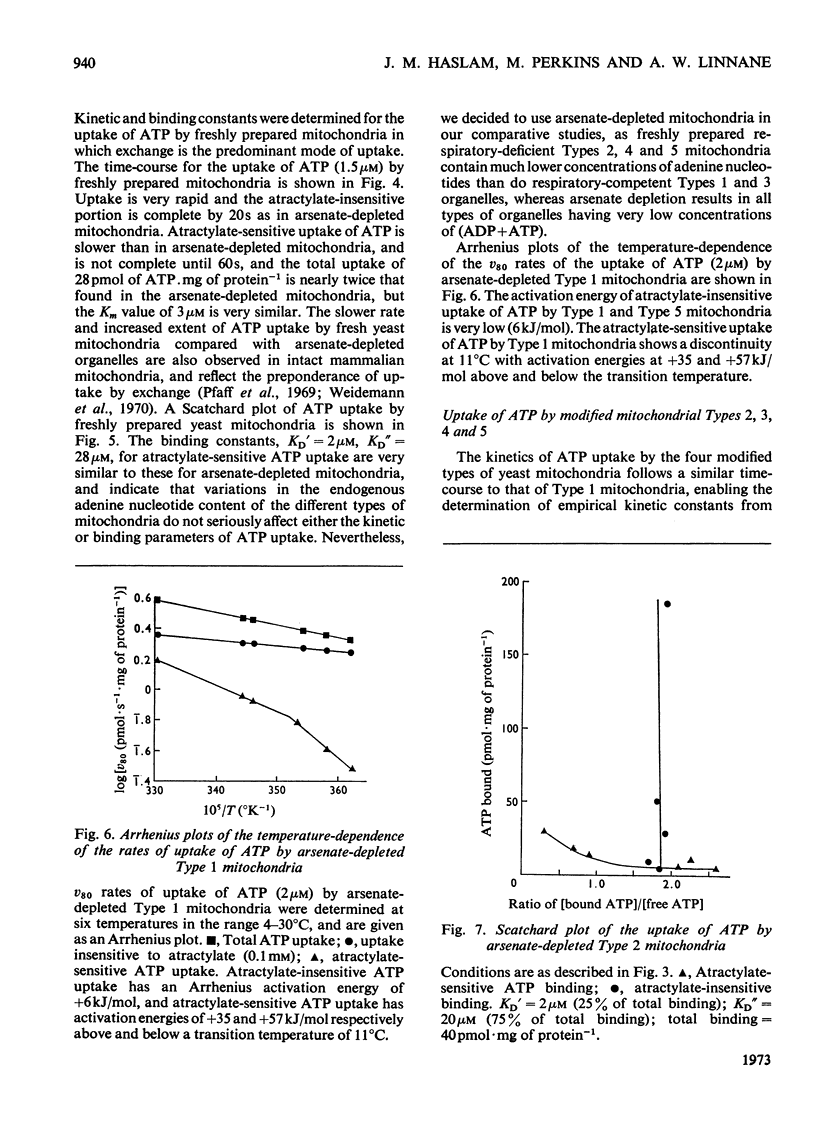
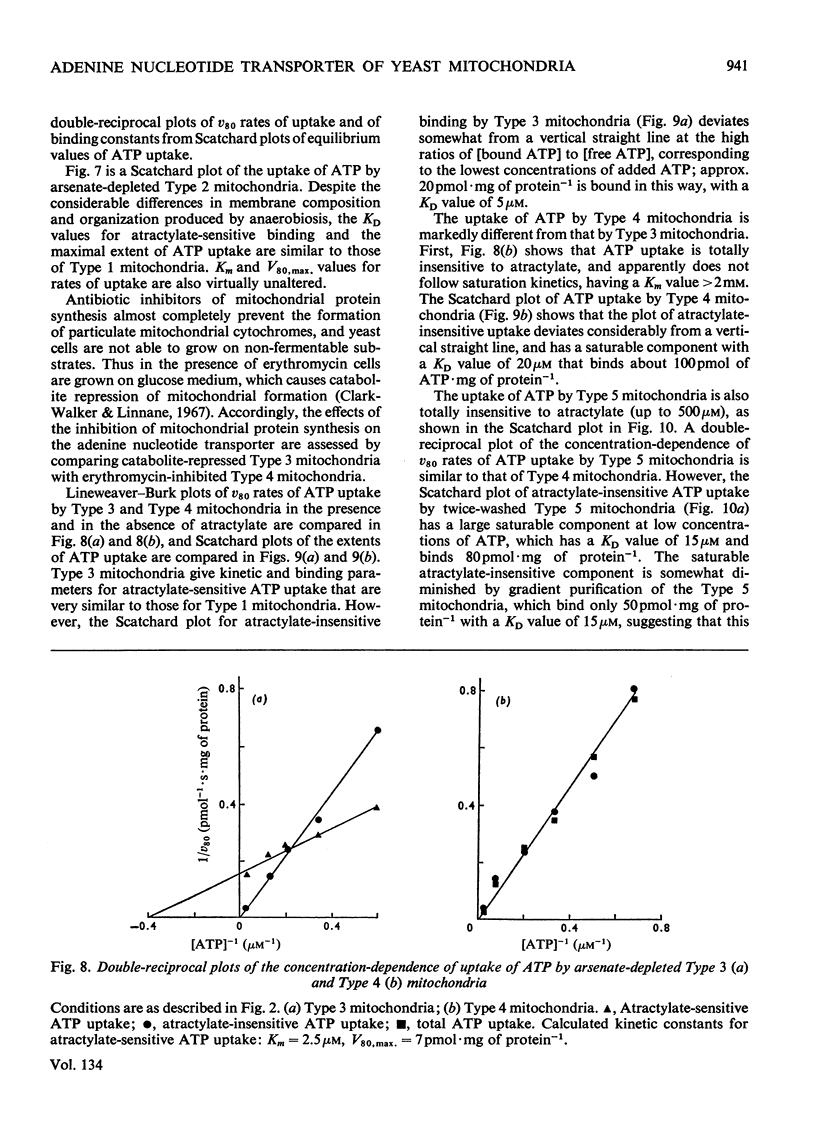
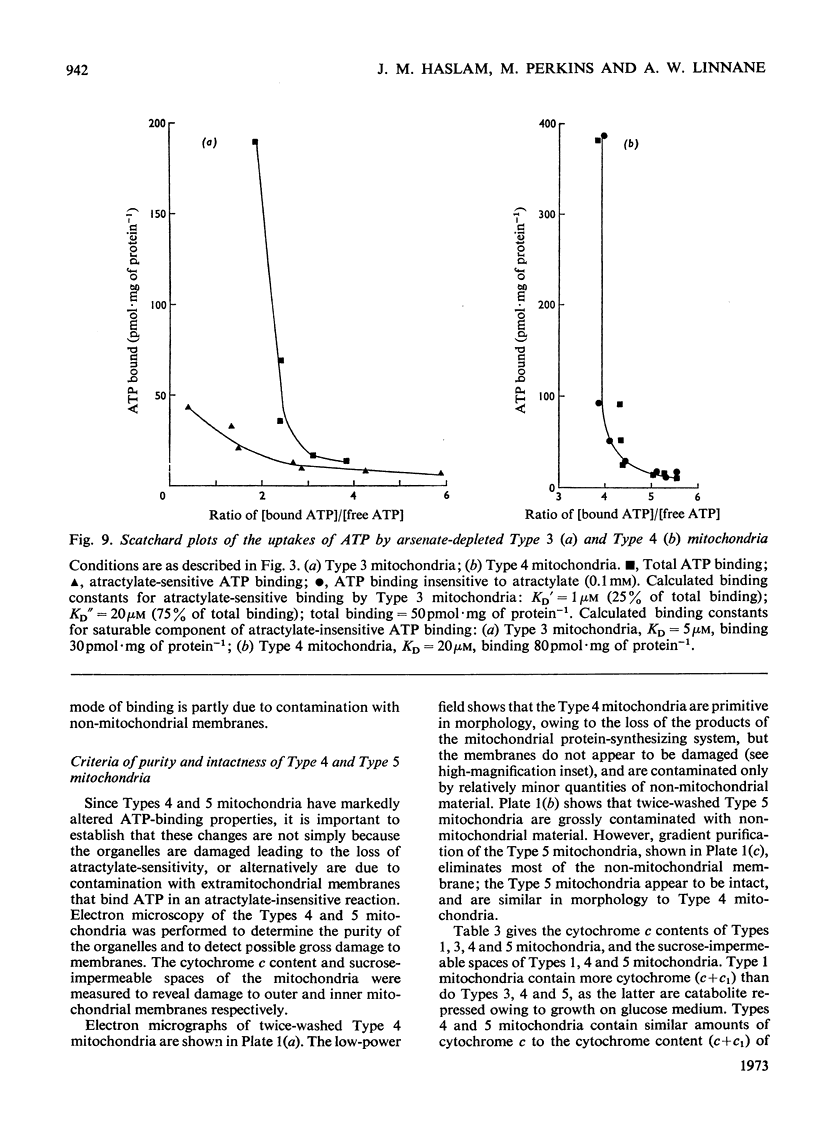
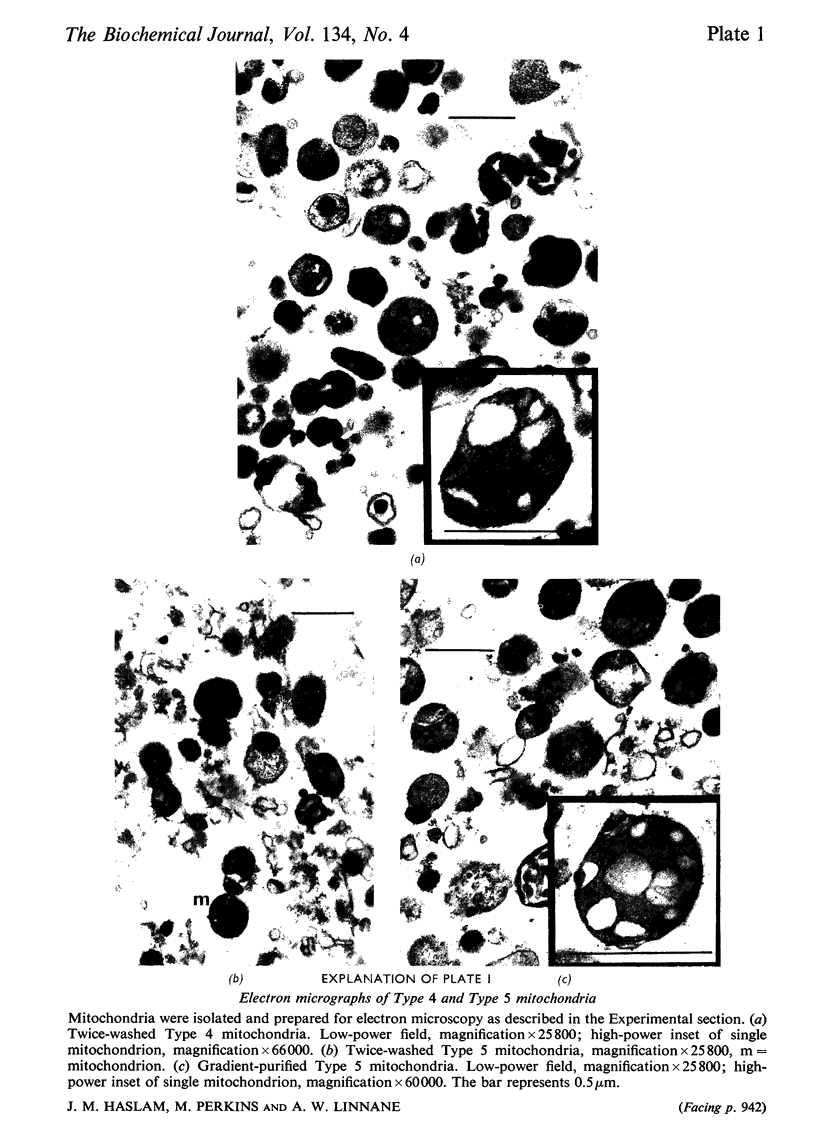
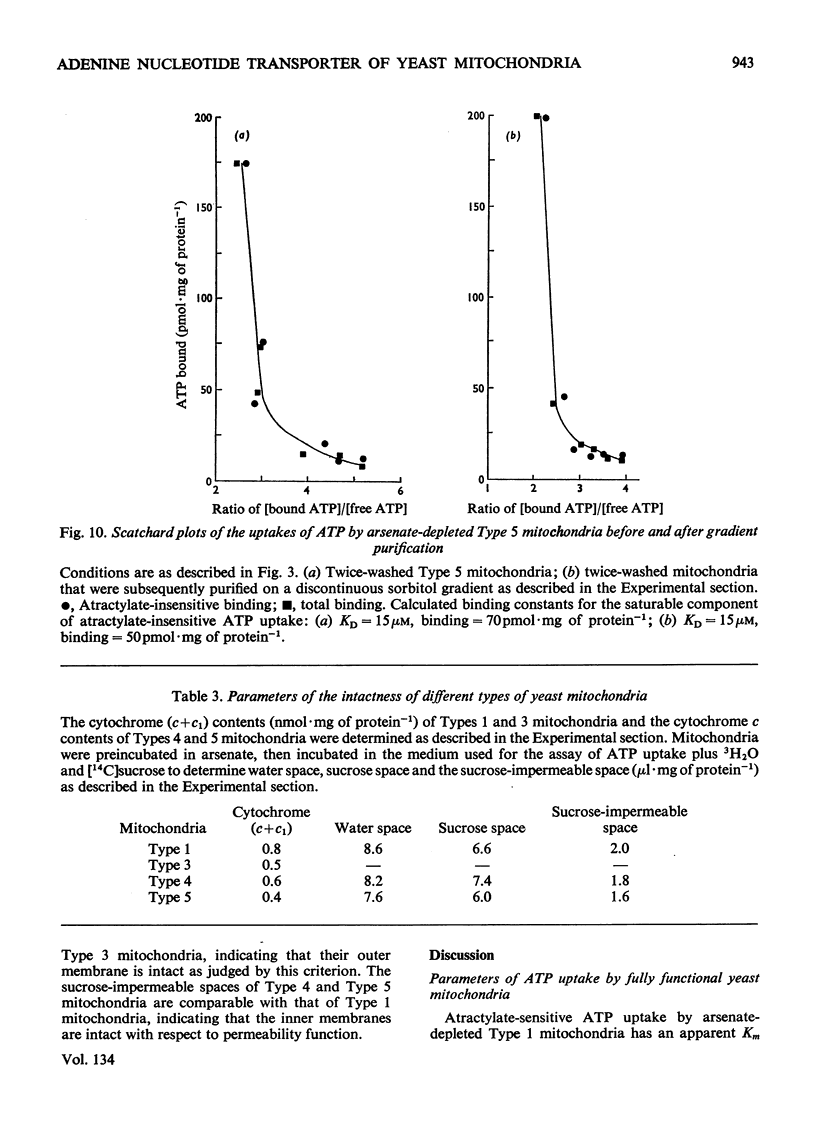
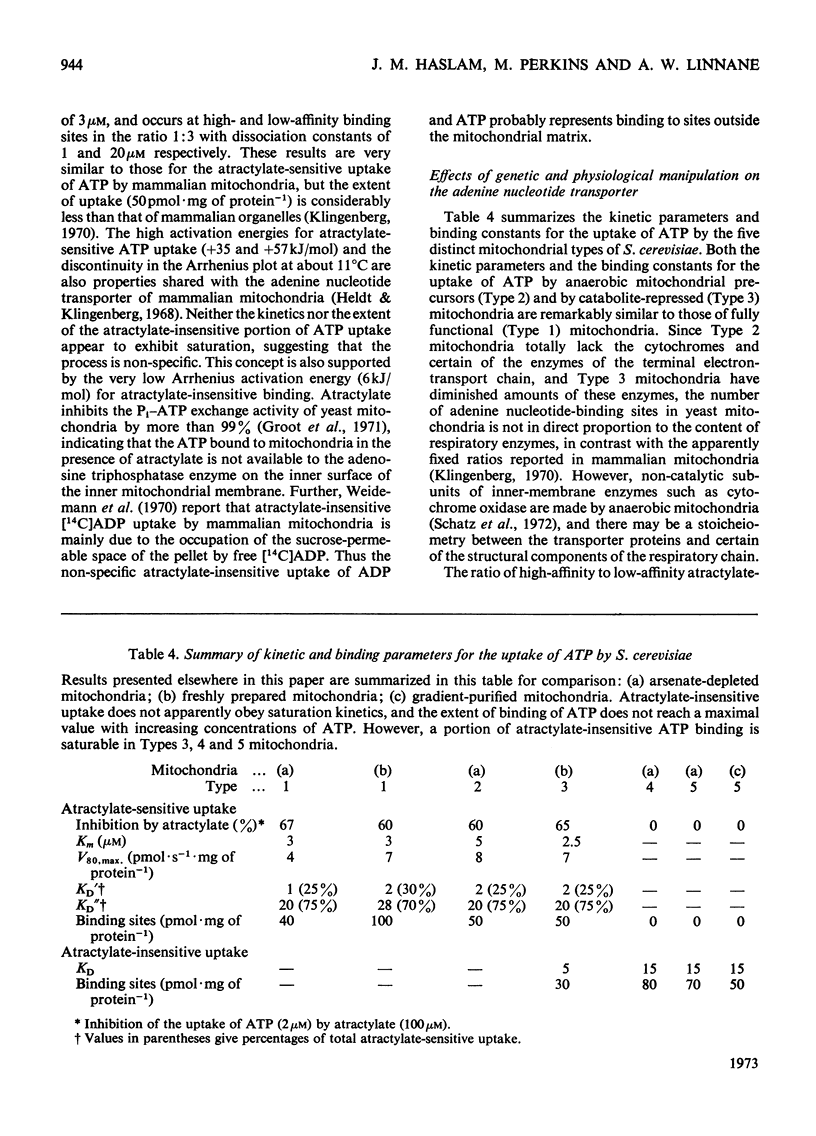
1. Parameters of ATP uptake by fully functional Saccharomyces cerevisiae mitochondria, including kinetic constants, binding constants and sensitivity to atractylate, closely resemble those of mammalian mitochondria. Scatchard plots of atractylate-sensitive adenine nucleotide binding indicate two distinct sites of high affinity (binding constant, KD′=1μm), and low affinity (binding constant, KD″=20μm) in the ratio 1:3. Uptake has high Arrhenius activation energies (+35 and +57kJ/mol), above and below a transition temperature of 11°C. Atractylate-insensitive ATP uptake is apparently not saturable and has a low Arrhenius activation energy (6kJ/mol), suggesting a non-specific binding process. 2. Kinetic and binding constants for ATP uptake are not significantly changed in catabolite-repressed or anaerobic mitochondrial structures. 3. Inhibition of the mitochondrial protein-synthesizing system by growth of cells in the presence of erythromycin, or loss of mitochondrial DNA by mutation profoundly alters the adenine nucleotide transporter. ATP uptake becomes completely insensitive to atractylate, and the high-affinity binding site is lost. However, the adenine nucleotide transporter does not appear to be totally eliminated, as a moderate amount of saturable low-affinity ATP binding remains. 4. It is concluded that products of the mitochondrial protein-synthesizing system, probably coded by mitochondrial DNA, are required for the normal function of the adenine nucleotide transporter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNI A., LUCIANI S., CONTESSA A. R. INHIBITION BY ATRACTYLOSIDE OF THE BINDING OF ADENINE-NUCLEOTIDES TO RAT-LIVER MITOCHONDRIA. Nature. 1964 Mar 21;201:1219–1220. doi: 10.1038/2011219a0. [DOI] [PubMed] [Google Scholar]
- Chavin S. I. Isolation and study of functional membrane proteins Present status and future prospects. FEBS Lett. 1971 May 20;14(5):269–282. doi: 10.1016/0014-5793(71)80278-8. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot G. S., Kovác L., Schatz G. Promitochondria of anaerobically grown yeast. V. Energy transfer in the absence of an electron transfer chain. Proc Natl Acad Sci U S A. 1971 Feb;68(2):308–311. doi: 10.1073/pnas.68.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M. Differences between the reactivity of endogenous and exogenous adenine nucleotides in mitochondria as studied at low temperature. Eur J Biochem. 1968 Mar;4(1):1–8. doi: 10.1111/j.1432-1033.1968.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Kolarov J., Subík J., Kovac L. Oxidative phosphorylation in yeast. 8. Osmotic and permeability properties of mitochondria isolated from wild-type yeast and from a respiration-deficient mutant. Biochim Biophys Acta. 1972 Jun 23;267(3):457–464. doi: 10.1016/0005-2728(72)90173-9. [DOI] [PubMed] [Google Scholar]
- Kolarov J., Subík J., Kovac L. Oxidative phosphorylation in yeast. IX. Modification of the mitochondrial adenine nucleotide translocation system in the oxidative phosphorylation-deficient mutant op 1 . Biochim Biophys Acta. 1972 Jun 23;267(3):465–478. doi: 10.1016/0005-2728(72)90174-0. [DOI] [PubMed] [Google Scholar]
- Kuzela S., Grecná E. Lack of amino acid incorporation by isolated mitochondria from respiratory-deficient cytoplasmic yeast mutants. Experientia. 1969;25(7):776–777. doi: 10.1007/BF01897625. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Onishi T., Kröger A., Heldt H. W., Pfaff E., Klingenberg M. The response of the respiratory chain and adenine nucleotide system to oxidative phosphorylation in yeast mitochondria. Eur J Biochem. 1967 May;1(3):301–311. doi: 10.1007/978-3-662-25813-2_41. [DOI] [PubMed] [Google Scholar]
- Perkins M., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. The effects of physiological and genetic manipulation of Saccharomyces cerevisiae on the mitochondrial transport systems for tricarboxylate-cycle anions. Biochem J. 1973 Aug;134(4):923–934. doi: 10.1042/bj1340923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M., Haslam J. M., Linnane A. W. The effects of physiological and genetic manipulation on the anion transport systems of yeast mitochondria. FEBS Lett. 1972 Sep 15;25(2):271–274. doi: 10.1016/0014-5793(72)80501-5. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Heldt H. W., Klingenberg M. Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem. 1969 Oct;10(3):484–493. doi: 10.1111/j.1432-1033.1969.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Heldt H. W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- Schatz G., Groot G. S., Mason T., Rouslin W., Wharton D. C., Salitzgaber J. Biogenesis of mitochondrial inner membranes in bakers' yeast. Fed Proc. 1972 Jan-Feb;31(1):21–29. [PubMed] [Google Scholar]
- Schatz G., Saltzgaber J. Protein synthesis by yeast promitochondria in vivo. Biochem Biophys Res Commun. 1969 Dec 4;37(6):996–1001. doi: 10.1016/0006-291x(69)90230-7. [DOI] [PubMed] [Google Scholar]
- Subík J., Kolarov J., Kovác L. Obligatory requirement of intramitochondrial ATP for normal functioning of the eucaryotic cell. Biochem Biophys Res Commun. 1972 Oct 6;49(1):192–198. doi: 10.1016/0006-291x(72)90028-9. [DOI] [PubMed] [Google Scholar]
- Watson K., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 13. The isolation of mitochondrial structures from anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1970 Jul;46(1):88–96. doi: 10.1083/jcb.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Erdelt H., Klingenberg M. Adenine nucleotide translocation of mitochondria. Identification of carrier sites. Eur J Biochem. 1970 Oct;16(2):313–335. doi: 10.1111/j.1432-1033.1970.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Lehninger A. L. The atractyloside-sensitive nucleotide binding site in a membrane preparation from rat liver mitochondria. J Biol Chem. 1968 Jun 10;243(11):3000–3008. [PubMed] [Google Scholar]




