Abstract
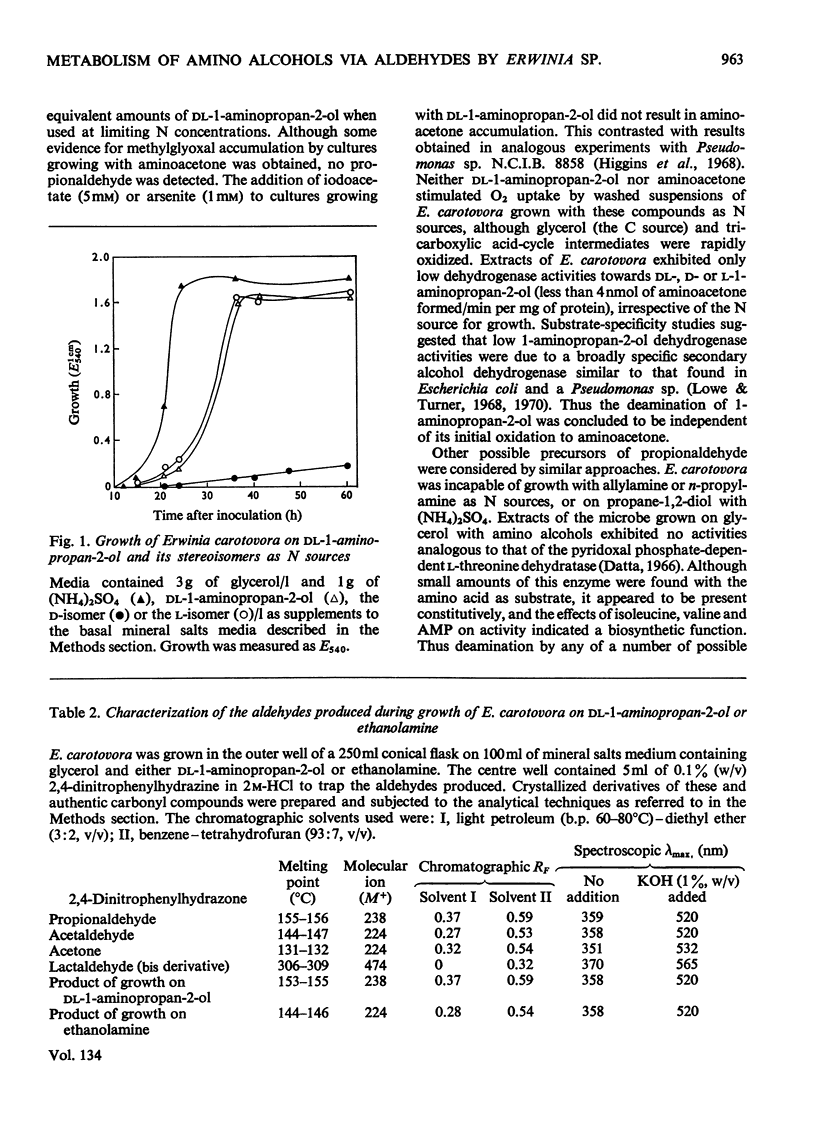
1. Growth of Erwinia carotovora N.C.P.P.B. 1280 on media containing 1-aminopropan-2-ol compounds or ethanolamine as the sole N source resulted in the excretion of propionaldehyde or acetaldehyde respectively. The inclusion of (NH4)2SO4 in media prevented aldehyde formation. 2. Growth, microrespirometric and enzymic evidence implicated amino alcohol O-phosphates as aldehyde precursors. An inducibly formed ATP–amino alcohol phosphotransferase was partially purified and found to be markedly stimulated by ADP, unaffected by NH4+ ions and more active with ethanolamine than with 1-aminopropan-2-ol compounds. Amino alcohol O-phosphates were deaminated by an inducible phospho-lyase to give the corresponding aldehydes. This enzyme, separated from the kinase during purification, was more active with ethanolamine O-phosphate than with 1-aminopropan-2-ol O-phosphates. Activity of the phospho-lyase was unaffected by a number of possible effectors, including NH4+ ions, but its formation was repressed by the addition of (NH4)2SO4 to growth media. 3. E. carotovora was unable to grow with ethanolamine or 1-aminopropan-2-ol compounds as sources of C, the production of aldehydes during utilization as N sources being attributable to the inability of the microbe to synthesize aldehyde dehydrogenase. 4. Of seven additional strains of Erwinia examined similar results were obtained only with Erwinia ananas (N.C.P.P.B. 441) and Erwinia milletiae (N.C.P.P.B. 955).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. II. Requirement for a cobamide coenzyme by an ethanolamine deaminase. J Biol Chem. 1965 Dec;240(12):4675–4681. [PubMed] [Google Scholar]
- Datta P. Purification and feedback control of threonine deaminase activity of Rhodopseudomonas spheroides. J Biol Chem. 1966 Dec 25;241(24):5836–5844. [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. O-phosphorylethanolamine ammonia lyase, a new pyridoxal phosphate-dependent enzyme. Biochem Biophys Res Commun. 1969 Jul 7;36(1):110–118. doi: 10.1016/0006-291x(69)90656-1. [DOI] [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. The metabolism of O-phosphorylethanolamine in animal tissues. I. O-phosphorylethanolamine phospho-lyase: partial purification and characterization. J Biol Chem. 1970 Sep 10;245(17):4414–4420. [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. The metabolism of O-phosphorylethanolamine in animal tissues. II. Metabolic regulation of O-phosphorylethanolamine phospho-lyase in vivo. Arch Biochem Biophys. 1970 Dec;141(2):423–429. doi: 10.1016/0003-9861(70)90158-x. [DOI] [PubMed] [Google Scholar]
- Grula M. M., Smith R. W., Parham C. F., Grula E. A. Cell division in a species of Erwinia. XI. Some aspects of the carbon and nitrogen nutrition of Erwinia species. Can J Microbiol. 1968 Nov;14(11):1217–1224. doi: 10.1139/m68-204. [DOI] [PubMed] [Google Scholar]
- Haq A., Dawes E. A. Pyruvic acid metabolism and ethanol formation in Erwinia amylovora. J Gen Microbiol. 1971 Nov;68(3):295–306. doi: 10.1099/00221287-68-3-295. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Pickard M. A., Turner J. M. Aminoacetone formation and utilization by pseudomonads grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):105–114. doi: 10.1099/00221287-54-1-105. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B. Aldehyde oxidation. I. Dehydrogenase from Pseudomonas fluorescens. J Biol Chem. 1958 May;232(1):75–87. [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols via aldehydes. J Gen Microbiol. 1971 Aug;67(3):379–381. doi: 10.1099/00221287-67-3-379. [DOI] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols. 1-Aminopropan-2-ol and ethanolamine metabolism via propionaldehyde and acetaldehyde in a species of Pseudomonas. Biochem J. 1973 May;134(1):167–182. doi: 10.1042/bj1340167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E., CHELDELIN V. H. Oxidation of acetaldehyde by Acetobacter suboxydans. J Biol Chem. 1956 May;220(1):177–191. [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. I. Purification, assay, and properties of the enzyme. J Biol Chem. 1968 Apr 25;243(8):1787–1793. [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. II. Physical and chemical properties and interaction with cobamides and ethanolamine. J Biol Chem. 1968 Apr 25;243(8):1794–1803. [PubMed] [Google Scholar]
- Lowe D. A., Turner J. M. Enzymic oxidation of D-1-aminopropan-2-ol by diol dehydrogenases of microbial origin. Biochim Biophys Acta. 1968 Dec 23;170(2):455–456. doi: 10.1016/0304-4165(68)90034-2. [DOI] [PubMed] [Google Scholar]
- Lowe D. A., Turner J. M. Origin of the D-1-aminopropan-2-ol fragment of vitamin B12. J Gen Microbiol. 1970 Nov;64(1):119–122. doi: 10.1099/00221287-64-1-119. [DOI] [PubMed] [Google Scholar]
- NARROD S. A., JAKOBY W. B. METABOLISM OF ETHANOLAMINE. AN ETHANOLAMINE OXIDASE. J Biol Chem. 1964 Jul;239:2189–2193. [PubMed] [Google Scholar]
- Pickard M. A., Higgins I. J., Turner J. M. Purification and properties of l-1-aminopropan-2-ol. NAD oxidoreductase from a pseudomonad grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):115–126. doi: 10.1099/00221287-54-1-115. [DOI] [PubMed] [Google Scholar]
- SPRINSON D. B., COULON A. The precursors of sphingosine in brain tissue. J Biol Chem. 1954 Apr;207(2):585–592. [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M. Microbial metabolism of amino ketones. L-1-aminopropan-2-ol dehydrogenase and L-threonine dehydrogenase in Escherichia coli. Biochem J. 1967 Jul;104(1):112–121. doi: 10.1042/bj1040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


