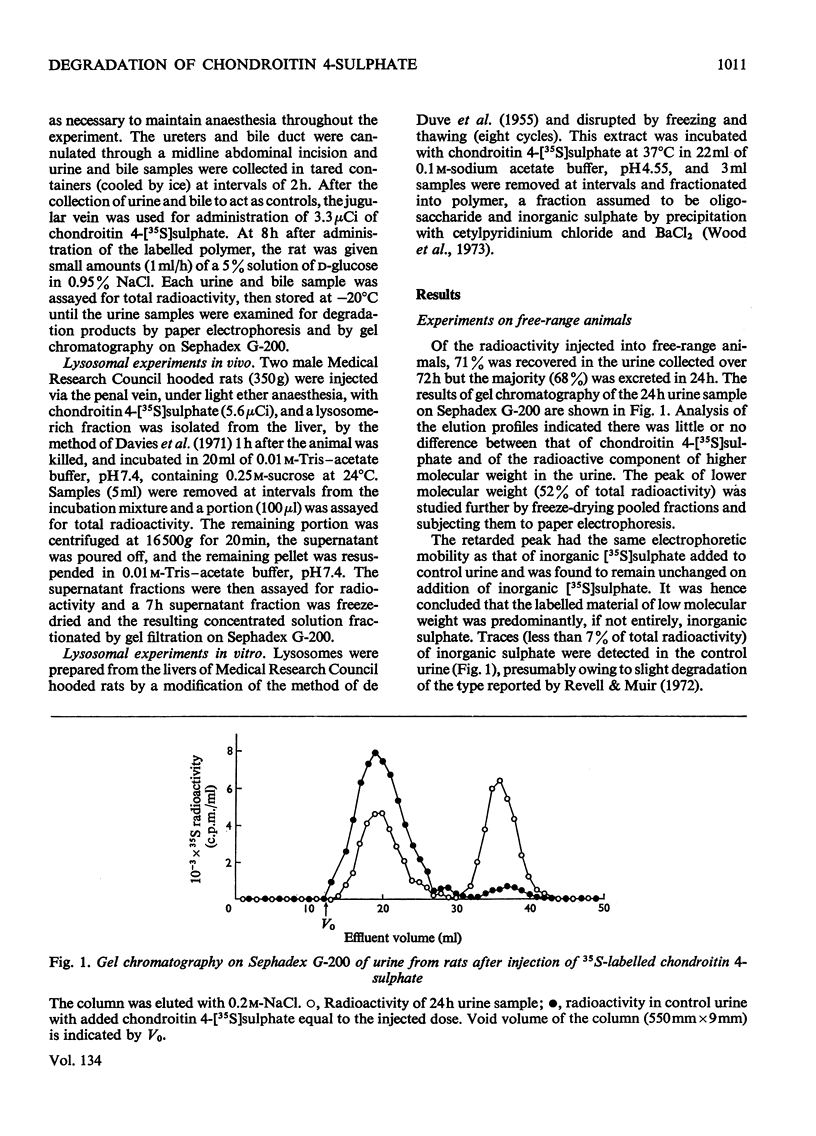
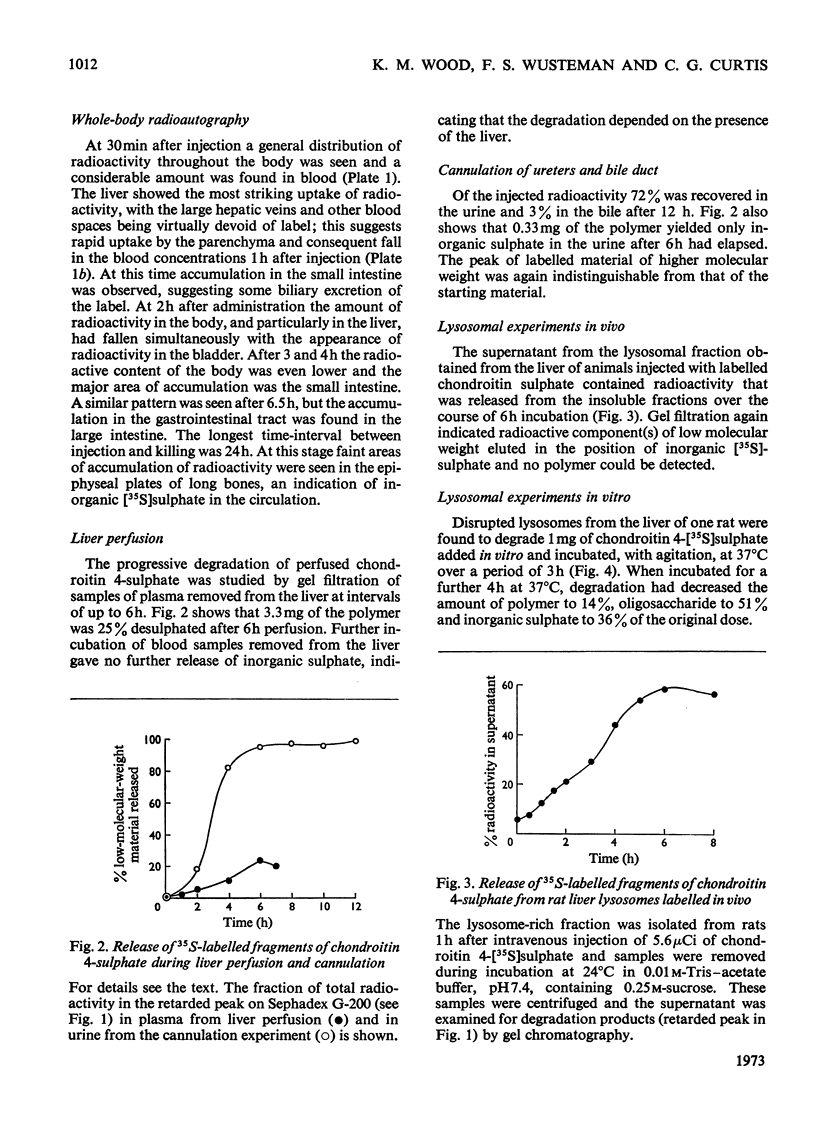
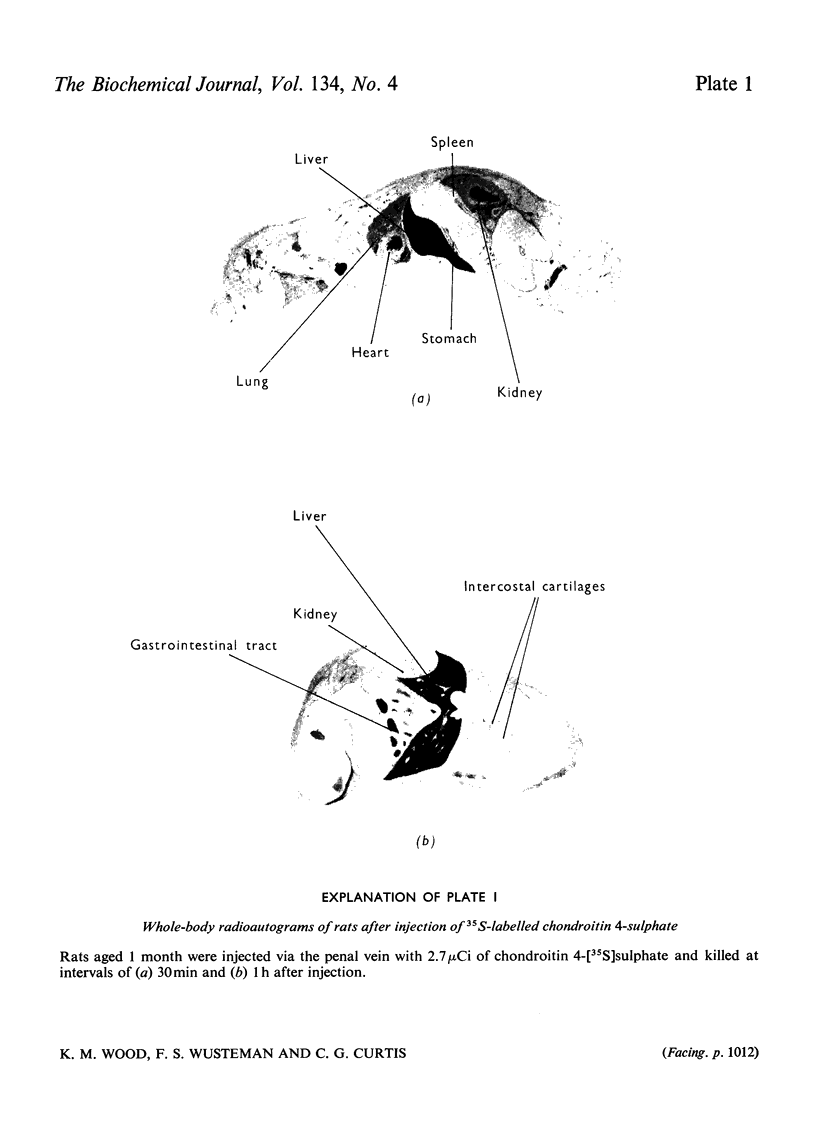
Abstract
The degradation of chondroitin 4-[35S]sulphate isolated from chick-embryo cartilage was studied in the rat by experiments on free-range animals, on wholly anaesthetized animals with ureter cannulae, by perfusion of isolated liver, by whole-body radioautography and by isolation of liver lysosomes. After injection into rats 68% of the radioactivity was recovered in the urine after 24h, approximately one-half of this being in the form of low-molecular-weight material, chiefly inorganic sulphate. Cannulation experiments demonstrated that the proportion of low-molecular-weight components excreted in the urine increased with time until, after 12h, virtually all was inorganic sulphate. Whole-body radioautography identified the liver as the major site of radioisotope accumulation after injection of labelled polysaccharide. Perfusion through isolated liver indicated that this organ has the ability to metabolize the polymer with the release of low-molecular-weight products, principally inorganic sulphate. Incubation of a lysosomal fraction prepared from rat liver after injection of chondroitin 4-[35S]sulphate gave rise to degradation products of low molecular weight, and experiments in vitro with rat liver lysosomes confirmed that these organelles are capable of the entire degradative process from chondroitin sulphate to free inorganic sulphate.
Full text
PDF

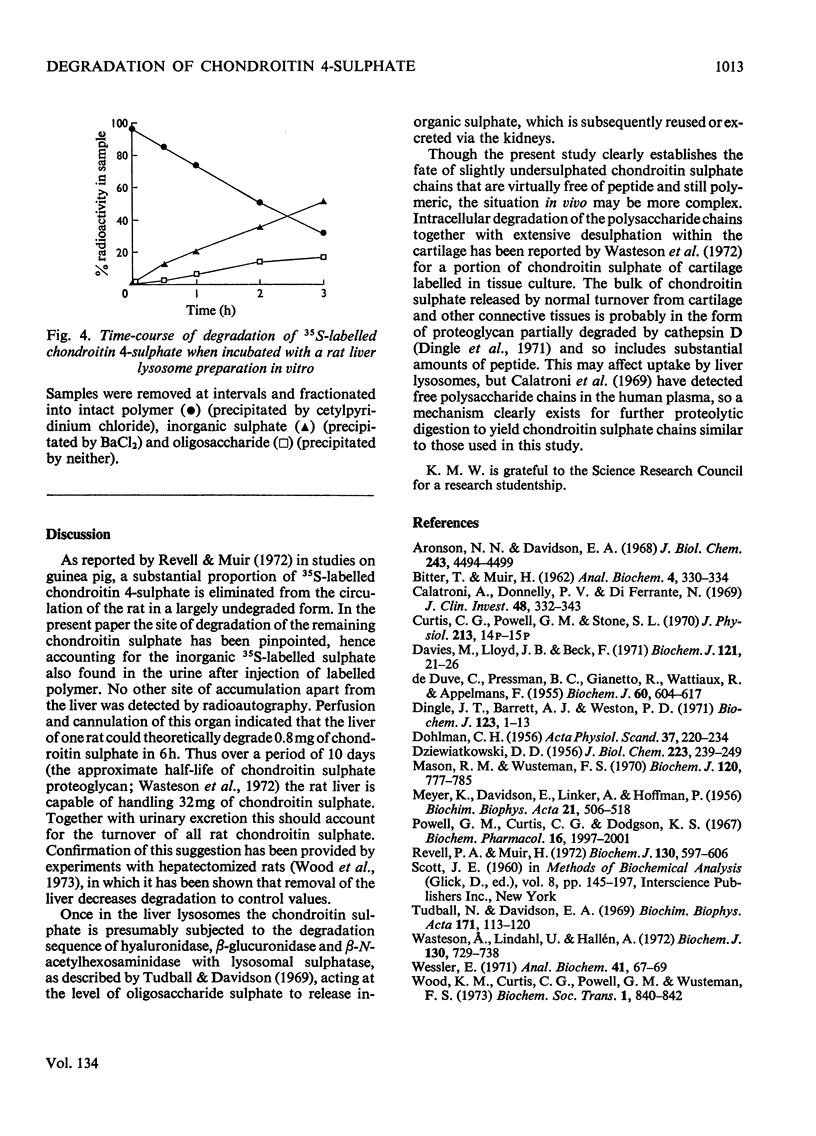
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Davidson E. A. Catabolism of mucopolysaccharides by rat liver lysosomes in vivo. J Biol Chem. 1968 Sep 10;243(17):4494–4499. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Calatroni A., Donnelly P. V., Di Ferrante N. The glycosaminoglycans of human plasma. J Clin Invest. 1969 Feb;48(2):332–343. doi: 10.1172/JCI105989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON E., HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 1956 Sep;21(3):506–518. doi: 10.1016/0006-3002(56)90188-3. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHLMAN C. H. The fate of the sulfate group of chondroitin sulfate after administration to rats. Acta Physiol Scand. 1956 Sep 26;37(2-3):220–234. doi: 10.1111/j.1748-1716.1956.tb01358.x. [DOI] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Some aspects of the metabolism of chondroitin sulfate-S35 in the rat. J Biol Chem. 1956 Nov;223(1):239–249. [PubMed] [Google Scholar]
- Davies M., Lloyd J. B., Beck F. The effect of Trypan Blue, suramin and aurothiomalate on the breakdown of 125 I-labelled albumin within rat liver lysosomes. Biochem J. 1971 Jan;121(1):21–26. doi: 10.1042/bj1210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. M., Wusteman F. S. The glycosaminoglycans of human tracheobronchial cartilage. Biochem J. 1970 Dec;120(4):777–785. doi: 10.1042/bj1200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. M., Curtis C. G., Dodgson K. S. The distribution of 35S-labelled sulphuric acid esters administered to mice and rats. Biochem Pharmacol. 1967 Oct;16(10):1997–2001. doi: 10.1016/0006-2952(67)90311-5. [DOI] [PubMed] [Google Scholar]
- Revell P. A., Muir H. The excretion and degradation of chondroitin 4-sulphate administered to guinea pigs as free chondroitin sulphate and as proteoglycan. Biochem J. 1972 Nov;130(2):597–606. doi: 10.1042/bj1300597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Tudball N., Davidson E. A. Isolation of a novel sulphatase from rat liver. Biochim Biophys Acta. 1969 Jan 7;171(1):113–120. doi: 10.1016/0005-2744(69)90110-7. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Lindahl U., Hallén A. Mode of degradation of the chondroitin sulphate proteoglycan in rat costal cartilage. Biochem J. 1972 Dec;130(3):729–738. doi: 10.1042/bj1300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]