Abstract
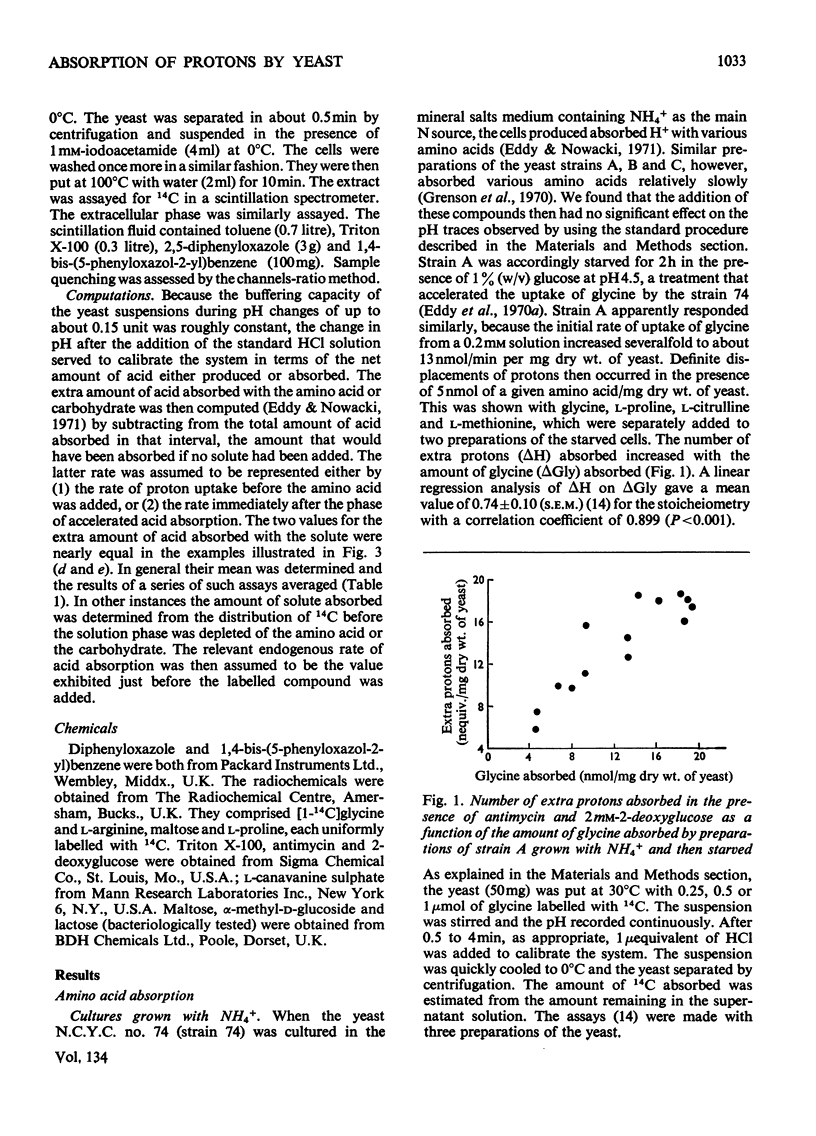
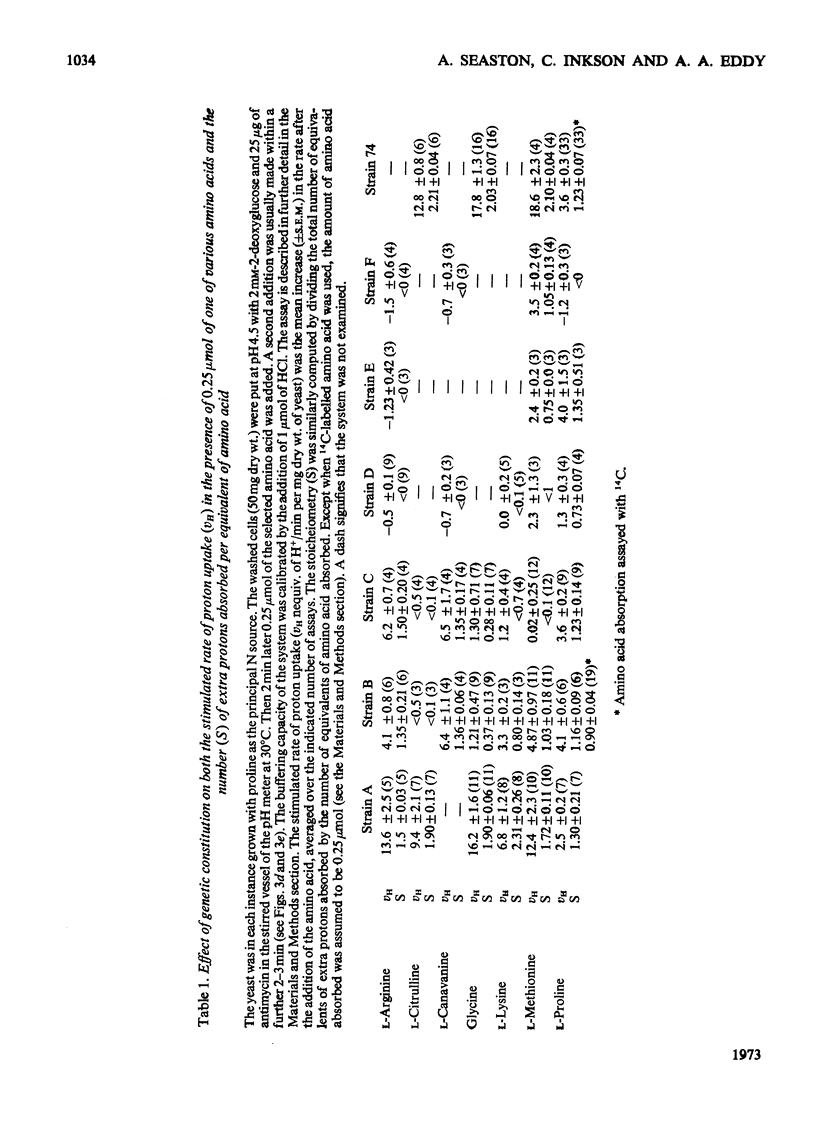
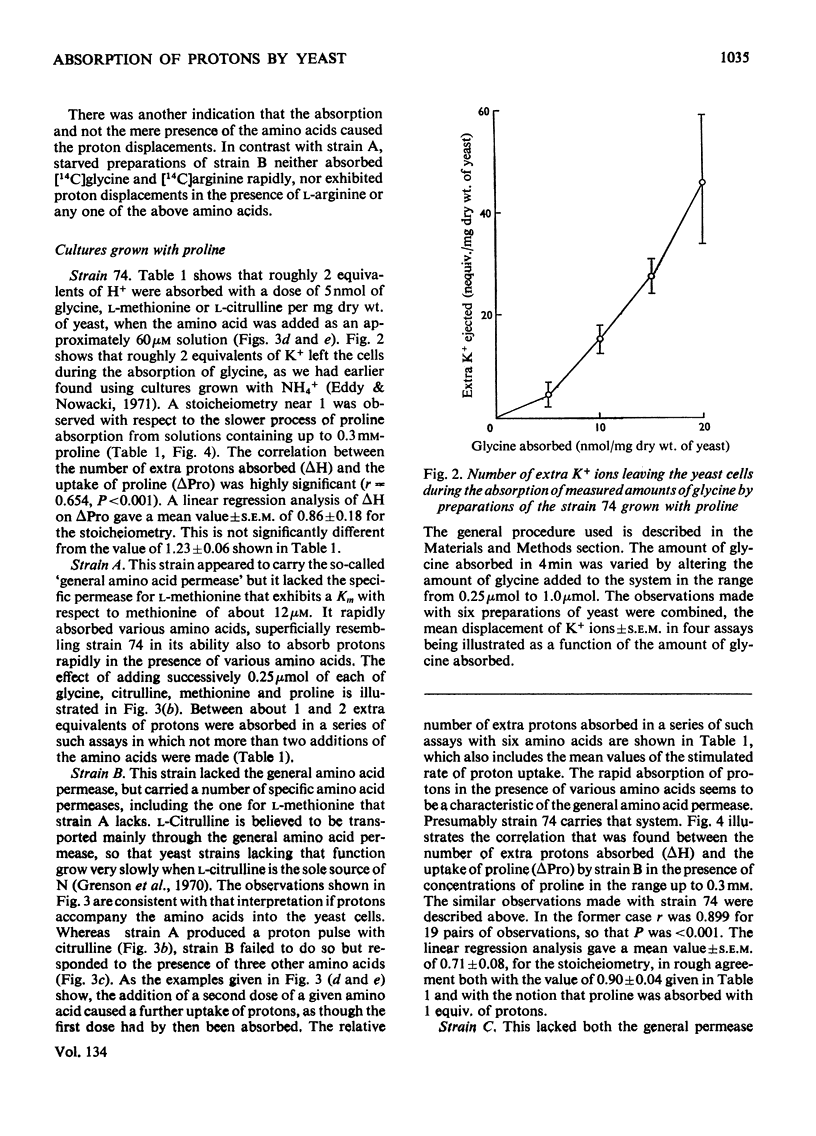
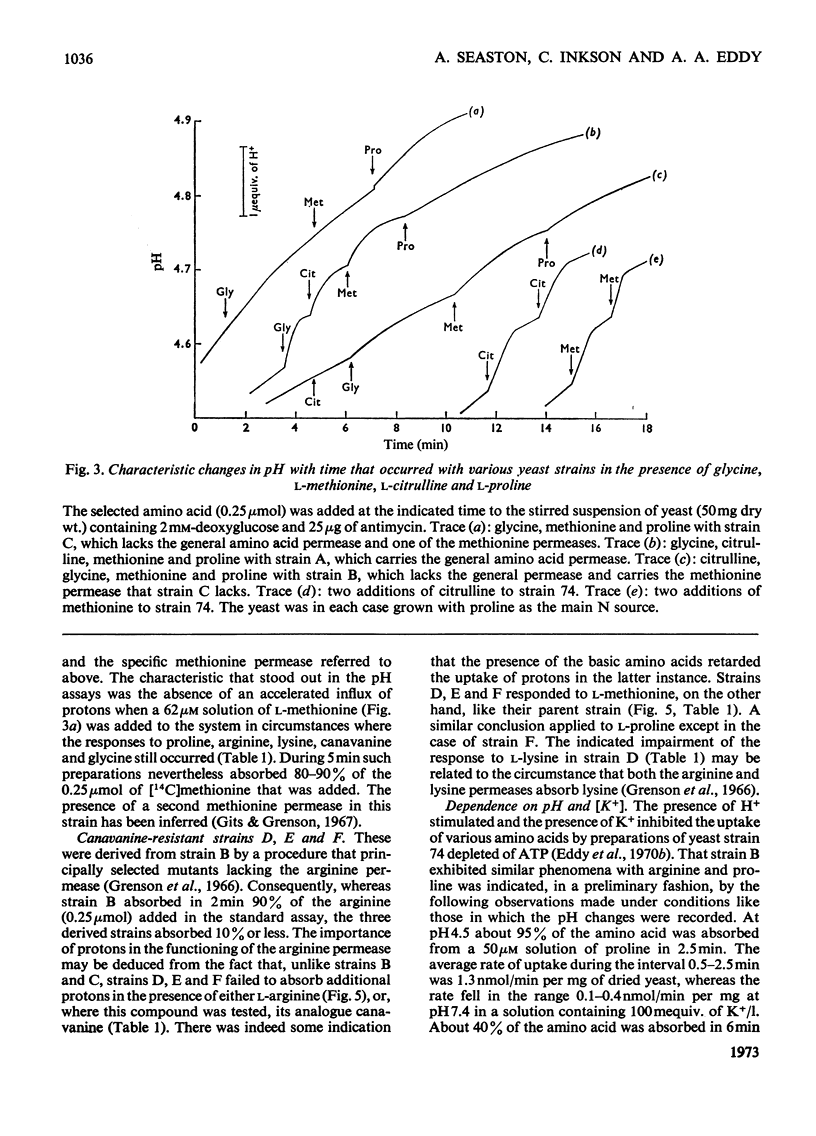
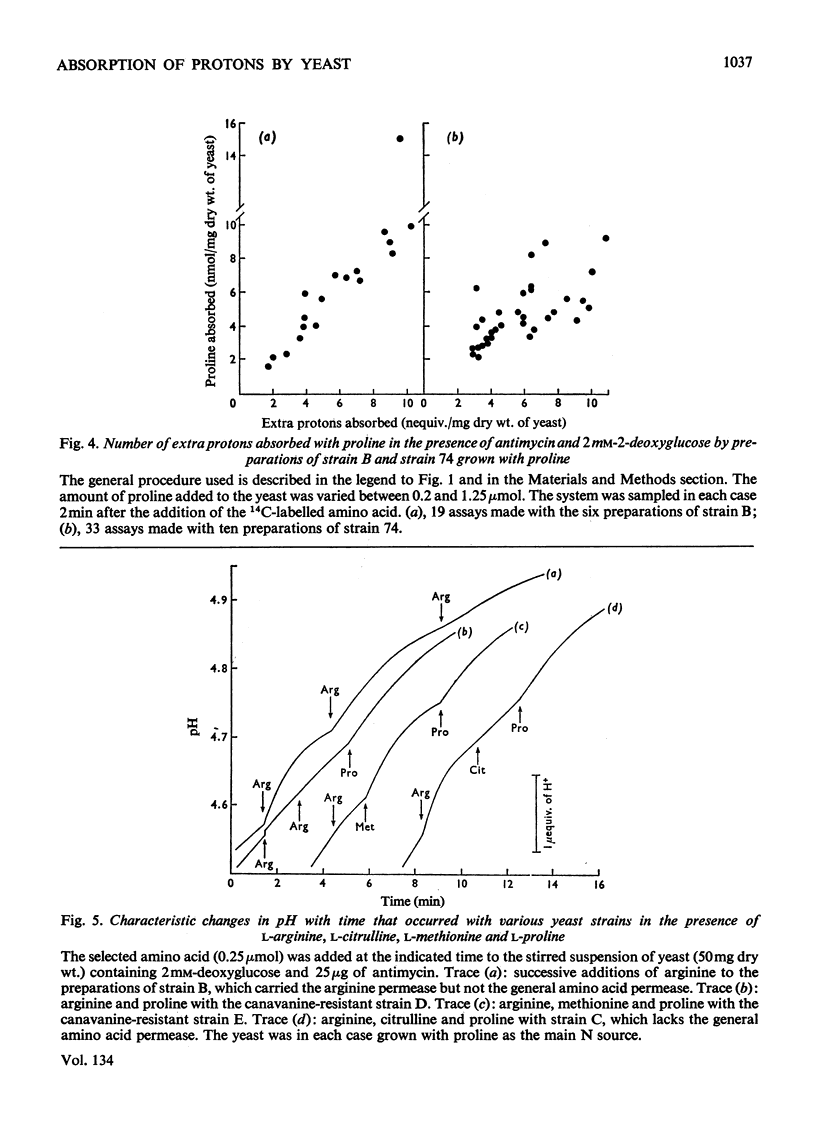
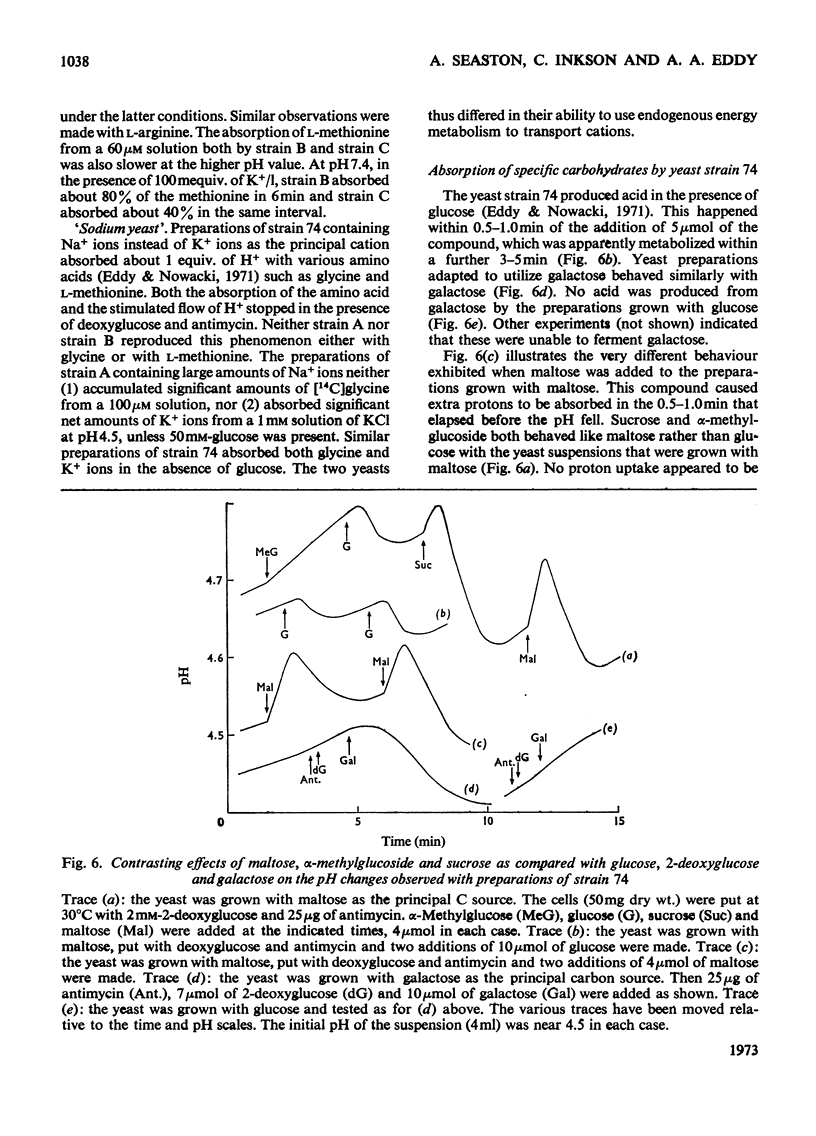
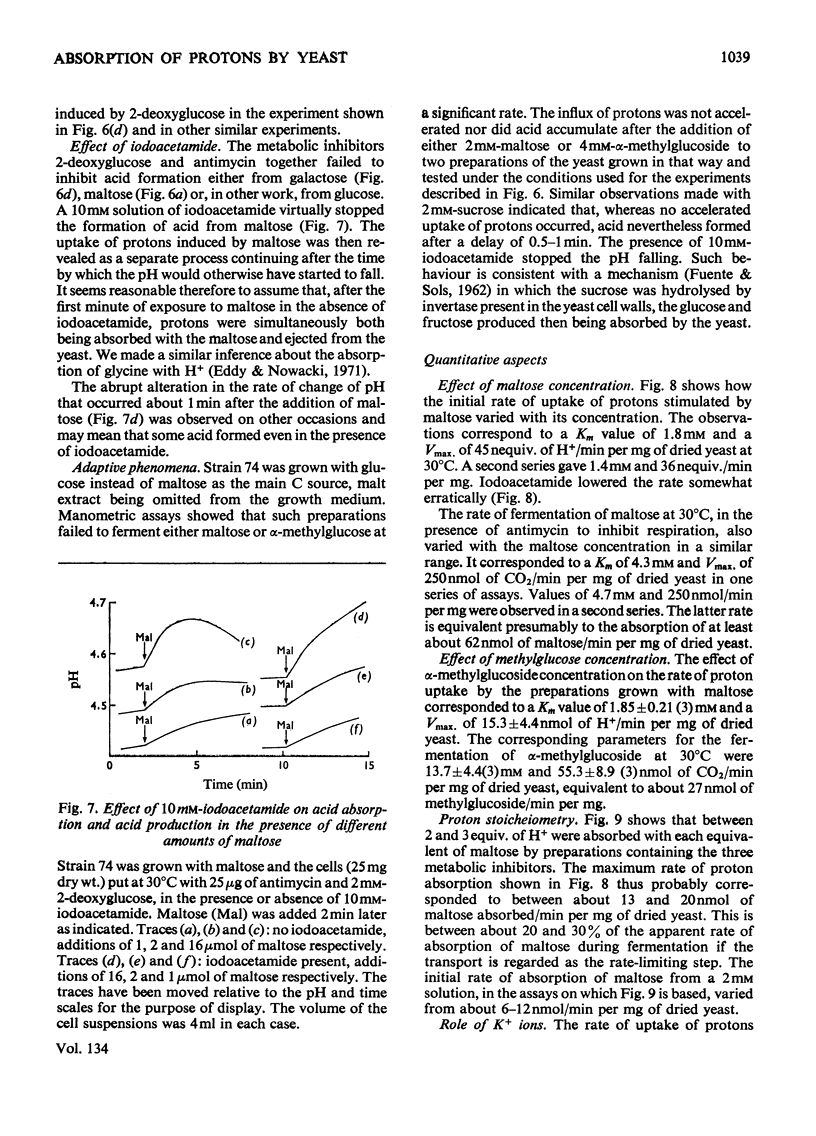
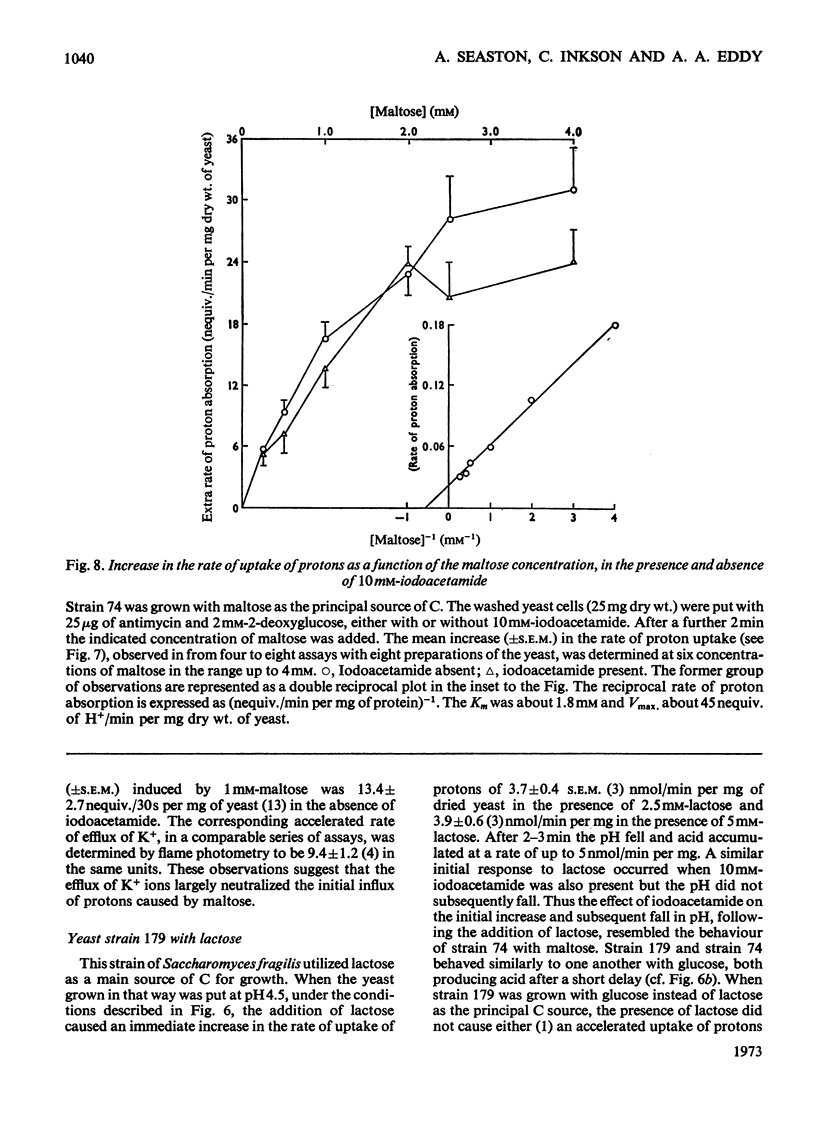
1. Proton uptake in the presence of various amino acids was studied in washed yeast suspensions containing deoxyglucose and antimycin to inhibit energy metabolism. A series of mutant strains of Saccharomyces cerevisiae with defective amino acid permeases was used. The fast absorption of glycine, l-citrulline and l-methionine through the general amino acid permease was associated with the uptake of about 2 extra equivalents of protons per mol of amino acid absorbed, whereas the slower absorption of l-methionine, l-proline and, possibly, l-arginine through their specific permeases was associated with about 1 proton equivalent. l-Canavanine and l-lysine were also absorbed with 1–2 equivalents of protons. 2. A strain of Saccharomyces carlsbergensis behaved similarly with these amino acids. 3. Preparations of the latter yeast grown with maltose subsequently absorbed it with 2–3 equivalents of protons. The accelerated rate of proton uptake increased up to a maximum value with the maltose concentration (Km=1.6mm). The uptake of protons was also faster in the presence of α-methylglucoside and sucrose, but not in the presence of glucose, galactose or 2-deoxyglucose. All of these compounds except the last could cause acid formation. The uptake of protons induced by maltose, α-methylglucoside and sucrose was not observed when the yeast was grown with glucose, although acid was then formed both from sucrose and glucose. 4. A strain of Saccharomyces fragilis that both fermented and formed acid from lactose absorbed extra protons in the presence of lactose. 5. The observations show that protons were co-substrates in the systems transporting the amino acids and certain of the carbohydrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G. Accumulation of trehalose and sucrose in relation to the metabolism of alpha-glucosides in yeasts of defined genotype. Biochim Biophys Acta. 1960 May 6;40:124–134. doi: 10.1016/0006-3002(60)91322-6. [DOI] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- Eddy A. A. A net gain of sodium ions and a net loss of potassium ions accompanying the uptake of glycine by mouse ascites-tumour cells in the presence of sodium cyanide. Biochem J. 1968 Jun;108(2):195–206. doi: 10.1042/bj1080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A. A sodium ion concentration gradient formed during the absorption of glycine by mouse ascites-tumour cells. Biochem J. 1969 Nov;115(3):505–509. doi: 10.1042/bj1150505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Backen K., Watson G. The concentration of amino acids by yeast cells depleted of adenosine triphosphate. Biochem J. 1970 Dec;120(4):853–858. doi: 10.1042/bj1200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Nowacki J. A. Stoicheiometrical proton and potassium ion movements accompanying the absorption of amino acids by the yeast Saccharomyces carlsbergensis. Biochem J. 1971 May;122(5):701–711. doi: 10.1042/bj1220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Llewellin J. M. The role of hydrogen and potassium ions in the transport of acidic amino acids in Staphylococcus aureus. Biochim Biophys Acta. 1972 Apr 14;266(1):182–205. doi: 10.1016/0005-2736(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Gibb L. E., Eddy A. A. An electrogenic sodium pump as a possible factor leading to the concentration of amino acids by mouse ascites-tumour cells with reversed sodium ion concentration gradients. Biochem J. 1972 Oct;129(4):979–981. doi: 10.1042/bj1290979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gits J. J., Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. 3. Evidence for a specific methionine-transporting system. Biochim Biophys Acta. 1967 Jul 3;135(3):507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hennaut C. Mutation affecting activity of several distinct amino acid transport systems in Saccharomyces cerevisiae. J Bacteriol. 1971 Feb;105(2):477–482. doi: 10.1128/jb.105.2.477-482.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- HARRIS G., THOMPSON C. C. The uptake of nutrients by yeasts. III. The maltose permease of a brewing yeast. Biochim Biophys Acta. 1961 Sep 2;52:176–183. doi: 10.1016/0006-3002(61)90915-5. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Energy expenditure is obligatory for the downhill transport of galactosides. J Mol Biol. 1971 Aug 14;59(3):447–459. doi: 10.1016/0022-2836(71)90309-3. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Höfer M. Uphill transport of sugars in the yeast Rhodotorula gracilis. Biochim Biophys Acta. 1965 Jul 22;102(2):410–422. doi: 10.1016/0926-6585(65)90131-7. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Cirillo V. P. Galactose transport in Saccharomyces cerevisiae. 3. Characteristics of galactose uptake in transferaseless cells: evidence against transport-associated phosphorylation. J Bacteriol. 1970 Sep;103(3):679–685. doi: 10.1128/jb.103.3.679-685.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS M. J., PHAFF H. J. RELEASE OF NITROGENOUS SUBSTANCES BY BREWER'S YEAST. IV. ENERGETICS IN SHOCK EXCRETION OF AMINO ACIDS. J Bacteriol. 1965 Apr;89:960–966. doi: 10.1128/jb.89.4.960-966.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- OKADA H., HALVORSON H. O. UPTAKE OF ALPHA-THIOETHYL D-GLUCOPYRANOSIDE BY SACCHAROMYCES CEREVISIAE. II. GENERAL CHARACTERISTICS OF AN ACTIVE TRANSPORT SYSTEM. Biochim Biophys Acta. 1964 Mar 16;82:547–555. doi: 10.1016/0304-4165(64)90446-5. [DOI] [PubMed] [Google Scholar]
- RIGGS T. R., WALKER L. M., CHRISTENSEN H. N. Potassium migration and amino acid transport. J Biol Chem. 1958 Dec;233(6):1479–1484. [PubMed] [Google Scholar]
- ROBERTSON J. J., HALVORSON H. O. The components of maltozymase in yeast, and their behavior during deadaptation. J Bacteriol. 1957 Feb;73(2):186–198. doi: 10.1128/jb.73.2.186-198.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- van Steveninck J. The transport mechanism of -methylglucoside in yeast evidence for transport-associated phosphorylation. Biochim Biophys Acta. 1970 Jun 2;203(3):376–384. doi: 10.1016/0005-2736(70)90178-1. [DOI] [PubMed] [Google Scholar]


