ABSTRACT
Background
Schistosomiasis is a chronic neglected tropical disease and Ghana's second most prevalent helminth infection. The annual mass drug administration of praziquantel to school children is aimed at reducing disease morbidity as a public health problem.
Aim
The study aimed to assess the prevalence and hematological profile of urinary schistosomiasis in Sempoa after over a decade of consecutive Mass Drug Administration (MDA) of praziquantel.
Methods
A community‐based cross‐sectional study involving 126 participants was conducted. Schistosoma haematobium eggs were identified and quantified using the urine filtration‐microscopy technique. The hematological profile was analyzed using a fully automated 5‐part Sysmex XN‐350 (Sysmex Co, Germany) analyzer.
Results
The study recorded a prevalence of 21.6% that was significantly related to water contact activities. Schistosomiasis was significantly associated with lower levels of Red Blood Cell (RBC) indices; MCV (p < 0.001), MCHC (p < 0.001), and MCH (p = 0.01) with higher platelet, lymphocytes, and basophil counts compared to the uninfected. Heavy infection was significantly associated with lower hemoglobin levels. The study further reported microhaematuria as a sensitive and specific proxy diagnostic tool for field surveillance in endemic communities.
Conclusion
Urinary schistosomiasis accompanied by an altered hematological profile persists in Sempoa under preventive praziquantel. Future control interventions must consider an integrated approach of marrying behavioral change, with preventive chemotherapy and vector control.
Keywords: hematological profile, hematuria, risk factors, urogenital schistosomiasis
1. Introduction
Schistosomiasis is a neglected tropical parasitic disease caused by blood flukes in the genus Schistosoma. Globally, 251.4 million people live in endemic areas. Out of these numbers, 75.3 million have been treated across 78 countries (WHO, Schistosomiasis Report [1]). Urogenital and intestinal schistosomiasis are the two forms of schistosomiasis in sub‐Saharan Africa, with the respective causative agents being Schistosoma haematobium (SH) and S. mansoni [2]. Schistosomiasis affects over a million people in Ghana, ranked as the second most prevalent helminth infection after soil‐transmitted helminths (STH) [3]. In 2008, the Neglected Tropical Disease Program (NTDP) mapped and stratified endemic areas into high, moderate, and low‐risk implementation units [4]. Since 2008, multiple rounds of preventive chemotherapy with praziquantel have been administered according to WHO guidelines (Table 1) with support from other interventions such as Water, Sanitation, and Hygiene (WASH) to control the burden of infection. However, schistosomiasis remains endemic in almost every district in Ghana (NTDP Annual Report, 2021).
Table 1.
WHO guidelines for the treatment of schistosomiasis according to risk category (Source: WHO Schistosomiasis Progress Report [5]).
| Category | Prevalence | Action | |
|---|---|---|---|
| High‐risk | ≥ 50% | Treat all school‐age children once a year | Simultaneously treat all adults once a year |
| Moderate‐risk | ≥ 10% but < 50% | Treat all school‐age children once every two years | Also, treat adults considered to be at risk |
| Low‐risk | < 10% | Treat all school‐age children once every three years | Praziquantel should be available in dispensaries and clinics |
Hematuria is a major overt symptom of urogenital schistosomiasis. Anemia due to chronic hematuria in pupils leads to a lack of concentration in the classroom, absenteeism, school dropout, low intelligence quotient (IQ), and reduced workforce in adults [6]. Chronic schistosomiasis alters blood cell levels which may later normalize following praziquantel treatment [7]. Eosinophilia is also observed in acute schistosomiasis but is not a guiding principle for serological conversion [8]. In the mice model, platelets were protective, and thrombocythemia was observed as a predictor of portal hypertension in schistosomiasis [9, 10, 11].
Mass drug administration (MDA) of praziquantel (PZQ) offers effective chemotherapy for controlling schistosomiasis among school‐aged children in sub‐Saharan Africa [12]. Nonetheless, the adult population may keep the infectious cycle reactivated. Hematuria is the primary sign that prompts diagnostic requests usually with other complications in school‐aged children [13]. Asymptomatic patients may, however, go unnoticed by clinicians, especially in malaria co‐endemic communities. Shrouded by the dominance of malaria and similarities in clinical presentations, it is likely to relegate the helminth etiology [14].
Sempoa is a fishing community in the Kwahu East Municipality of Ghana and is ranked among the high‐risk implementation units of schistosomiasis. Contrary to the World Health Organization's (WHO) guidelines to simultaneously treat adults, the current MDA of praziquantel targets only school‐age children in school. Against this background, the study determined the persistence of urogenital schistosomiasis and associated the intensity of infection with changes in hematological profile. The study further examined the prevailing risk factors, the diagnostic utility of microhematuria, and hematological parameters as proxies in resource‐limited settings especially field surveys in the absence of microscopy.
2. Materials and Methods
2.1. Sampling and Study Design
This study was a cross‐sectional community‐hospital‐based study conducted across the four zones of Sempoa. One hundred and Fifty‐three (153) participants were randomly recruited at a sampling interval of 3. Out of this number, 126 met the inclusion criteria and were successfully enrolled, 8 participants refused to consent while 19 tested positive for malaria. Sampling was randomized based on a pre‐registered household and inhabitant list within the four clustered zones. A standard pre‐tested questionnaire was used to collect sociodemographic data and assess risk factors. All community entry protocols were duly followed. Sampling was done before the annual school‐based MDA of praziquantel.
2.2. Study Site
Sempoa is a community located in the Afram Basin of the Kwahu East Municipality of the Eastern Region of Ghana. It occupies a total land area of around 860 square kilometers. The community's population is estimated to be around 900 with children accounting for about 30% according to the Centre for Democratic Development's (CDD) report, 2019. Sempoa is situated in the low‐lying plains of the Afram River which serves as the major source of water for all livelihood activities (Figure 1). The prime occupations of the inhabitants are agriculture and fishing. The community is divided into four zones with varying proximity to the Afram River. The Ahenfo Medical Centre, a private healthcare facility, serves as the primary healthcare provider in addition to a public health center. The last school‐based MDA of praziquantel (PZQ) in Sempoa was conducted in 2019.
Figure 1.
GPS Coordinates showing the location of Sempoa and the Afram River (Generated live using the KoboCollect Data Collection App).
2.3. Ethical Considerations
Ethical and protocol approval was obtained from the Institutional Review Board (IRB) of Baldwin University College. Written informed consent and assent were obtained from each participant before being enrolled in the study.
2.4. Inclusion and Exclusion Criteria
Participants who were 5 years of age and above and had stayed in Sempoa for a year and above were included. Participants on praziquantel treatment, malaria‐related anemia, and other hemoglobinopathies such as leukemia, sickle cell anemia, and observed allergic reactions at the time of sampling were excluded. Menstruating females were also excluded.
2.5. Urine Collection and Parasitological Examination
Urine samples were collected in appropriately labeled leak‐proof sterile containers with screw lids. Complete voided urine was collected between the hours of 10:00 and 14:00 h Greenwich Mean Time (GMT) when egg excretion was known to be highest [15]. The samples were collected in batches on ice and immediately transported to the laboratory for processing. Samples were visually assessed for macrohematuria, followed by using the Urit 10 V multi‐stick urine reagent strips (Urit Medical Electronic Company Ltd) to detect microhaematuria. Egg/10 mL of filtered urine for S. haematobium was determined and categorized into low and high intensity per World Health Organisation (WHO) guidelines.
Quantification of egg load was determined by filtering 10 mL of urine through a 12.0 micron, 13 mm polycarbonate membrane filter (Steritech Cooperation). Trapped eggs were fixed on the microscope slide, stained with a drop of iodine, and observed under x10 and x40 objective lens (Light infection < 50 eggs/10 mL, heavy >/=50 eggs/10 mL).
2.6. Blood Sample Collection and Hematological Analysis
A 3 mL of venous blood was collected with a sterile vacutainer needle into an EDTA tube. Full blood count indices were estimated using a fully automated 5‐part Sysmex XN‐350 (Sysmex Co, Germany) analyzer. The hematological parameters were Hemoglobin (HB), mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), hematocrit (HCT), basophils (BAS), eosinophils (EOS), platelets (PLT), monocytes (MONO), neutrophils (NEUT), and lymphocytes (LYM).
2.7. Data Analysis
The analysis was performed using the Statistical Package for the Social Sciences (SPSS), Version 27. The raw data was tested for normality using the Kolmogorov‐Smirnov test. The ages were categorized and expressed in proportions of urogenital schistosomiasis status. Categorical variables were summarized by proportion and 95% confidence interval (CI); while quantitative variables were summarized by mean and standard error of the mean (SEM). The ratio of the odds was used to compare the strength of association between categorical variables and their Schistosomiasis status. Significant differences between continuous variables were compared using the independent Student's t‐test. One‐way ANOVA (analysis of variance) was used to compare the means of hematological profiles among negative, light, and heavy infections. ROC curves were constructed to determine the diagnostic performance of hematological parameters using the area under the curve (AUROC). The tests were two‐sided at a statistical significance level of less than 0.05 at 95% Confidence Interval (CI).
3. Results
3.1. Characteristics of Study Participants Regarding Schistosomiasis Infection
The average age of the participants was approximately 27 years with the majority falling between the ages of 15–30 years. Age, sex, number of years one has stayed in the community, and history of previous infection was not significantly associated with schistosomiasis infection. The number of infections was significantly lower (p = 0.02) in people who had a history of praziquantel intake (43.7%) compared to praziquantel naïve individuals (56.3%). Visible hematuria was observed in 20/29 of the positive cases (p < 0.001). The dipstick chemistry for microhematuria showed 86.7% (26/29) concordance with the microscopy result (p < 0.001) (Table 2).
Table 2.
Characteristics of the study participants regarding schistosomiasis infection status.
| Variable | Total N (%) | Schistosoma positive N (%) | Schistosoma negative N (%) | Odds ratio (95% Cl) | p‐value |
|---|---|---|---|---|---|
| Age (years) | |||||
| < 15 | 25 (19.8) | 6 (24.0) | 19 (76.0) | N/A | 0.975 |
| 15–30 | 51 (40.5) | 12 (23.5) | 39 (76.5) | ||
| > 30 | 50 (39.7) | 11 (22.0) | 39 (78.0) | ||
| Mean ± SD | 26.7 ± 9.4 | ||||
| Gender | |||||
| Male | 102 (81.0) | 22 (21.6) | 80 (78.4) | 1.49 (0.55–4.07) | 0.428 |
| Female | 24 (19.0) | 7 (29.2) | 17 (70.8) | ||
| Zone | |||||
| 4 | 57 (45.2) | 14 (24.6) | 43 (75.4) | 0.85 (0.37–1.96) | 0.708 |
| 6 | 69 (54.8) | 15 (21.7) | 54 (78.3) | ||
| Length of stay (years) | |||||
| 1–5 | 46 (36.5) | 11 (23.4) | 36 (76.6) | 0.97 (0.41–2.27) | 0.936 |
| > 5 | 79 (62.7) | 18 (22.8) | 61 (77.2) | ||
| History of hematuria | |||||
| No | 29 (23.0) | 7 (24.1) | 22 (75.9) | 0.92 (0.35–2.44) | 0.870 |
| Yes | 97 (77.0) | 22 (22.7) | 75 (77.3) | ||
| Treatment of hematuria | |||||
| No | 44 (34.9) | 11 (25.0) | 33 (75.0) | 0.84 (0.36–1.99) | 0.698 |
| Yes | 82 (65.1) | 18 (22.0) | 64 (78.0) | ||
| Painful urination | |||||
| No | 43 (34.1) | 9 (20.9) | 34 (79.1) | 1.19 (0.49–2.92) | 0.689 |
| Yes | 83 (65.9) | 20 (24.1) | 63 (75.9) | ||
| Past praziquantel intake | |||||
| No | 71 (56.3) | 22 (31.0) | 49 (69.0) | 3.08 (1.20–7.87) | 0.016 |
| Yes | 55 (43.7) | 7 (12.7) | 48 (87.3) | ||
| Visible hematuria | |||||
| No | 102 (81.0) | 9 (8.8) | 93 (91.2) | 51.7 (14–184.5) | 0.001 |
| Yes | 24 (19.0) | 20 (83.3) | 4 (16.7) | ||
| Schistosoma awareness | |||||
| No | 39 (30.9) | 5 (12.8) | 34 (87.2) | 2.59 (0.91–7.40) | 0.069 |
| Yes | 87 (69.1) | 24 (27.6) | 63 (72.4) | ||
| Dipstick reaction | |||||
| Negative | 96 (76.2) | 3 (3.1) | 93 (96.9) | 201 (42.39–957.77) | 0.001 |
| Positive | 30 (23.8) | 26 (86.7) | 4 (13.3) | ||
| Total | 126 (100) | 29 (23.0) | 97 (77.0) |
Note: Bold values are statistically significant p‐values.
3.2. Risk Factors and Hematological Profile of Schistosomiasis Infected Participants
The majority (82.8%) of the participants who engaged in at least, a water contact activity (swimming, washing, domestic use, fishing, irrigation) had schistosomiasis. The odds of schistosomiasis infection for water contact activity were 2.96 (p = 0.04) (Table 3). From the student T‐test analysis, mean levels of MCV, MCHC, and MCH were significantly higher in controls the uninfected individuals (controls) compared to the infected. The mean levels of platelet (PLT), LYM, and BAS were significantly higher in the infected compared to the uninfected (Table 4).
Table 3.
Water contact activities and risk of urinary schistosomiasis infection.
| Water contact activities | NEG n (%) | OR (95%CI) | p‐value | |
|---|---|---|---|---|
| POS n (%) | ||||
| Yes | 24 (82.8) | 5 (17.2) | 2.960 (1.041–7.576) | 0.043 |
| No | 60 (61.9) | 37 (38.1) |
Table 4.
Hematological profile of the study participants regarding schistosomiasis status.
| Parameters | Cases (N = 29) | Control (N = 97) | F‐statistic | p value |
|---|---|---|---|---|
| HCT | 41.67 ± 0.94 | 48.04 ± 0.49 | 0.04 | 0.846 |
| MCV | 73.09 ± 2.11 | 86.33 ± 0.48 | 63.71 | 0.0001 |
| MCHC | 29.65 ± 0.56 | 34.01 ± 0.14 | 48.25 | 0.0001 |
| MCH | 28.19 ± 0.57 | 30.59 ± 0.22 | 6.67 | 0.011 |
| HB (g/dL) | 10.04 ± 0.31 | 11.71 ± 0.19 | 2.04 | 0.156 |
| EOSx109/L | 2.02 ± 0.30 | 1.65 ± 0.93 | 0.25 | 0.621 |
| NEUT | 3.09 ± 0.23 | 2.43 ± 0.13 | 0.06 | 0.816 |
| BAS | 0.05 ± 0.01 | 0.04 ± 0.01 | 7.80 | 0.006 |
| LYM | 3.02 ± 0.26 | 2.11 ± 0.01 | 9.14 | 0.003 |
| MONO | 0.43 ± 0.06 | 0.49 ± 0.03 | 3.92 | 0.050 |
| PLT | 228.14 ± 14.24 | 178.21 ± 3.83 | 32.18 | 0.0001 |
3.3. Stratification of Hematological Profile Based on Heavy and Light Infections
When the positive cases were stratified according to heavy and light infections, the mean levels of Hemoglobin (HB) and NEUT in heavy infections were significantly lower (p < 0.001 and 0.01 respectively) compared to the uninfected. Both light and heavy‐infected participants showed significantly lower HCT, MCV, MCHC, and MCH levels compared to the uninfected. Light infections had significantly higher platelet and lymphocyte levels compared to the uninfected (Table 5).
Table 5.
Hematological profile of the study participants regarding S. haematobium infection stratified by heavy and light infections.
| Parameters | S. haematobium infection | Control (N = 97) | p‐value | |
|---|---|---|---|---|
| Heavy (N = 5) | Light (N = 24) | |||
| HCT | 38.40 ± 1.00 | 42.35 ± 1.07 | 48.04 ± 0.49 ab | 0.0001 |
| MCV | 69.05 ± 5.40 | 73.93 ± 2.31 | 86.33 ± 0.48 ab | 0.0001 |
| MCHC | 28.94 ± 1.58 | 29.80 ± 0.61 | 34.01 ± 0.14 ab | 0.0001 |
| MCH | 27.64 ± 1.62 | 28.30 ± 0.62 | 30.59 ± 0.22 ab | 0.0001 |
| HB (g/dl) | 10.04 ± 0.55 | 10.02 ± 0.36 | 11.71 ± 0.19 b | 0.0001 |
| EOSx10^9/l | 1.88 ± 0.47 | 2.05 ± 0.35 | 1.65 ± 0.93 | 0.997 |
| NEUT | 3.91 ± 0.41 | 2.92 ± 0.25 | 2.43 ± 0.13 b | 0.013 |
| BAS | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.334 |
| LYM | 3.05 ± 0.44 | 3.01 ± 0.30 | 2.11 ± 0.01 a | 0.0001 |
| MONO | 0.69 ± 0.04 c | 0.38 ± 0.06 | 0.49 ± 0.03 | 0.032 |
| PLT | 180.20 ± 10.63 c | 238.13 ± 16.40 | 178.21 ± 3.83 a | 0.0001 |
Note: Values are mean ± SEM. ‘ a ’ indicates a significant difference between the control and light S. haematobium infection, ‘ b ’ indicates a significant difference between the control and heavy S. haematobium infection, ‘ c ’ indicates a significant difference between light S. haematobium infection and heavy S. haematobium infection. Bold values are statistically significant p‐values.
3.4. Diagnostic Utility of Urine Dipstick, Macrohematuria, and Hematological Indices
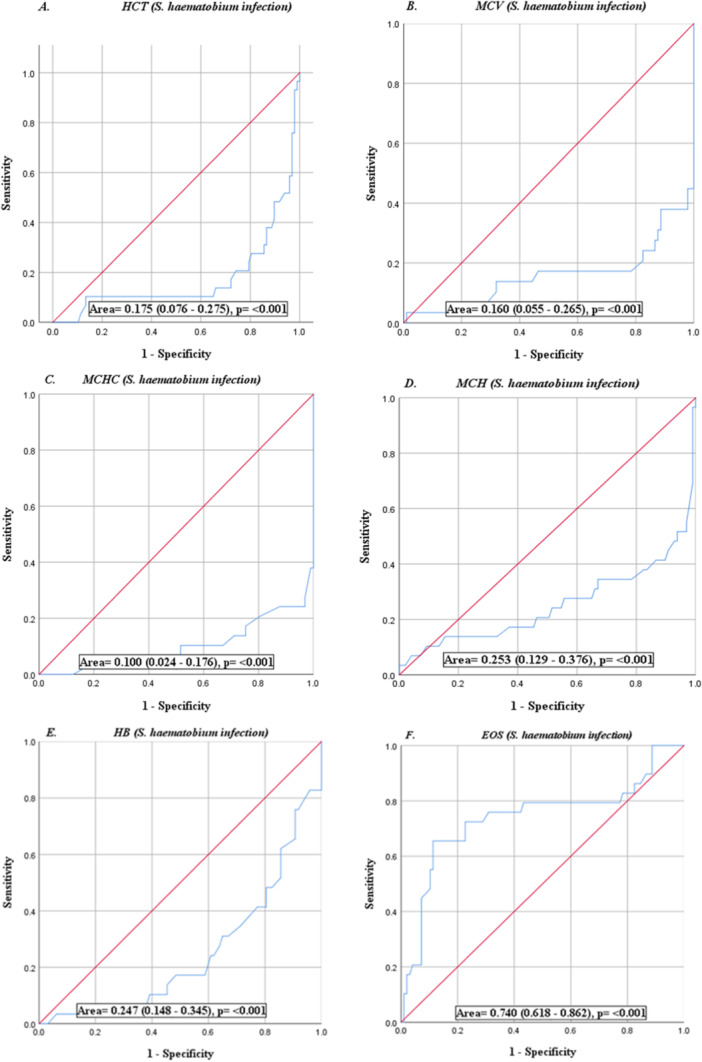
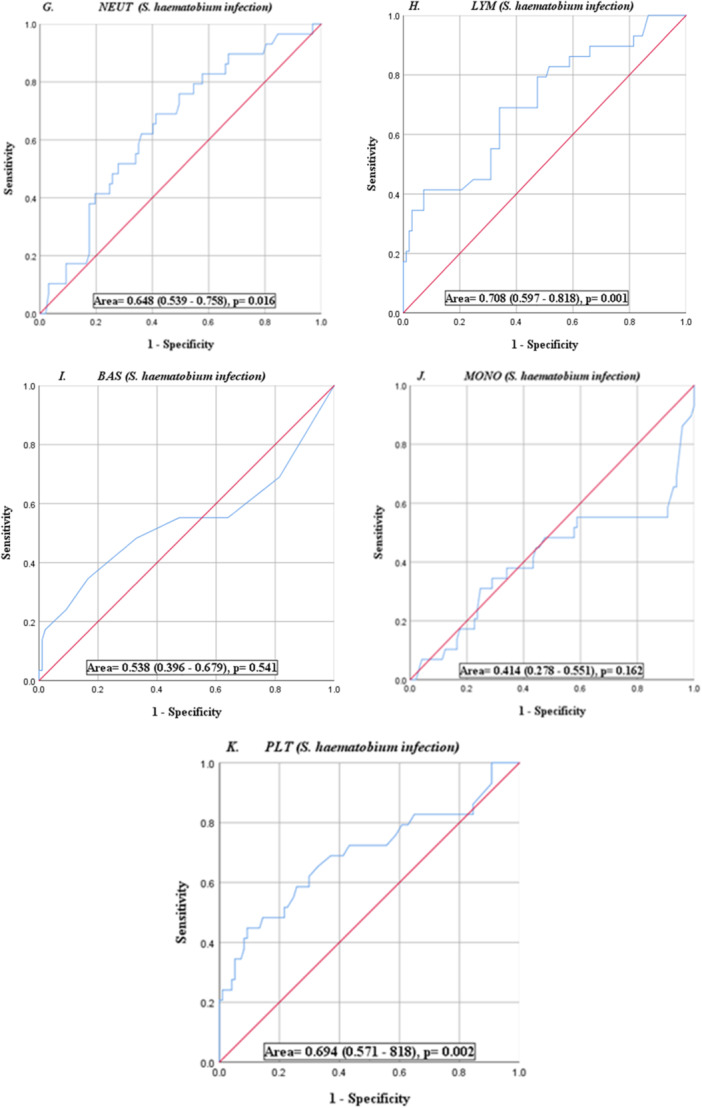
Using microscopy as the point of reference, the sensitivity and specificity of the urine dipstick test for microhaematuria showed a sensitivity of 95.9% and specificity of 89.7% in diagnosing schistosomiasis. The positive and negative predictive values were 96.9% and 86.7% respectively. The sensitivity of the macrohematuria was 69.0% and the specificity was 95.9% with negative and positive predictive values of 91.2% and 83.3% respectively (Table 6). But for EOS and lymphocytes, hematological parameters had poor diagnostic performance as shown by the Area Under the ROC (AUROC < 70%) curve (Figure 2).
Table 6.
Diagnostic performance of the urine dipstick and visible hematuria results relative to the presence of S. haematobium eggs.
| Schistosoma infection | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Dipstick | 95.9 | 89.7 | 96.9 | 86.7 |
| Visible hematuria | 69.0 | 95.9 | 83.3 | 91.2 |
Figure 2.
ROC curves (A‐K) show the accuracies of individual full blood parameters as diagnostic tools. Diagnostic performance is based on the area under the curve (AUROC).
4. Discussion
The epidemiological profile of schistosomiasis in endemic communities is expected to change considerably owing to the scale‐up of school‐based annual preventive chemotherapy with praziquantel. Baseline prevalence data from a nationwide mapping classified the Afram Basin of the Kwahu East municipality within the high‐risk category of schistosomiasis infection (Magalhaes et al. 2011). The World Health Organisation's (WHO) treatment guidelines for Schistosomiasis [5] recommend annual treatment for school‐aged children and at‐risk adults. Thus, the Neglected Tropical Diseases Control Program with support from its partners has scaled up MDA in endemic districts [16, 17]. We assessed the persistence of urinary schistosomiasis and its associated risk factors after over a decade of consecutive praziquantel MDA in Sempoa. We further evaluated the association between the hematological profile, infection intensity, and diagnostic utility of microhematuria as a surrogate for the urine filtration method in a resource‐limited setting. The difference in prevalence was insignificantly higher in females than males. This may reflect differences in anatomical makeup, gender norms, and associated water contact activities [18]. Furthermore, differences in urogenital schistosomiasis infection were not significant among participants who had stayed in an endemic community for at least a year. Water contact activities such as swimming, fishing, irrigation, and domestic use remain predominant which promote re‐infection and persistence of the disease [19]. Our findings suggest a significant reduction in infection among participants with a history of praziquantel intake compared to PZQ naïve participants. However, preventive chemotherapy alone has not produced a sustainable impact partly owing to the continuous engagement in water contact practices [20]. These activities are multifactorial and integrated into their source of livelihood and therefore schistosomiasis control strategies must consider a comprehensive One‐health approach. Our results showed a prevalence of 21.6% which falls within the moderate‐risk category (WHO guidelines for treatment of schistosomiasis [5]). In addition to engaging in water contact activities, the high prevalence could be attributed to the average adult population used in this study who were outside the school‐based MDA target.
The relationship between schistosomiasis and anemia has been extensively studied albeit with varying conclusions [7, 8, 12, 21]. Our result showed that participants with urinary schistosomiasis had significantly lower MCV, MCHC, MCH, and HB levels compared to SH uninfected people. Hemoglobin levels were significantly lower in heavy infections compared to the uninfected. In addition, our results show that people in schistosomiasis endemic areas may generally have lower hemoglobin levels compared to international reference ranges. This agrees with previous studies which also reported low RBC indices in urinary schistosomiasis [21]. Hypochromic microcytic anemia could be attributed to iron deficiency due to extra‐corporeal loss, splenic sequestration, and inflammation [22]. Consistent with other studies, the increase in eosinophil, lymphocytes, and basophil levels suggests gross systemic immune response and hypersensitivity reactions to adult schistosomes and their eggs [23]. The significantly higher platelet levels from the study could be a negative feedback mechanism to compensate for the incessant loss of blood that characterizes urogenital schistosomiasis.
The utility of microhematuria could serve as a rapid diagnostic tool for urinary schistosomiasis in endemic areas where microscopy is unavailable [24]. We report a significant association between microhematuria and urinary schistosomiasis with a high Sensitivity and Specificity. Similar findings were reported by Bogoch et al. [25] in Northern Ghana and Kosinski et al. [26] among Ghanaian school children. Talab et al. (2018) showed high sensitivity and low specificity to hematobium infection for macrohematuria and dipstick. On the contrary, our results show low sensitivity of macrohematuria which may be due to the exclusion of menstruating participants, the adult population used, and the rapid processing of samples aimed at avoiding false positives owing to urine alkalinity. Our findings are further supported by Kotb et al. [27] who reported high sensitivity and specificity of microhaematuria to diagnose urinary schistosomiasis among school children in Egypt. However, urine dipstick for microhaematuria does not test for the presence of red blood cells but rather the peroxidase activity on erythrocytes. Therefore, using microhaematuria as an alternative for urinary schistosomiasis diagnosis must be quality controlled against confounding factors such as menstruating females, myoglobin levels, high doses of vitamin C, pH, and other environmental factors that could lead to either false positive or false negative results [28].
The study further revealed the diagnostic potential of the hematological indices in urinary schistosomiasis using the area under the ROC curve. Eosinophil, lymphocyte, and platelet showed moderate diagnostic potential with AUROC of 74.0%, 70.8% and 69.4% respectively.
4.1. Study Limitation
The analysis of our study did not differentiate between children and adults in terms of their S. hematobium egg carriage and hematological parameters. We recommend further studies to categorize and present case‐specific results for children and adults.
5. Conclusion
The persistence of urinary schistosomiasis after multiple rounds of praziquantel chemotherapy in Sempoa owns credence to the incessant engagements in water contact practices. Urinary schistosomiasis displays an altered hematological profile characterized by low RBC indices, which is mostly associated with heavy infection. The study considers the use of microhaematuria together with risk factor assessment for point‐of‐contact diagnosis of urinary schistosomiasis where microscopy is unavailable. Schistosomiasis control programs should apply a comprehensive One Health approach involving sociocultural dynamics, vector control, and MDA in school children and at‐risk adults.
Author Contributions
Alahaman Nana Boakye: conceptualization, investigation, funding acquisition, writing–original draft, methodology, validation, visualization, writing–review and editing, software, formal analysis, project administration, data curation, supervision, resources. Neuwell Hatsu: conceptualization, investigation, resources. Samuel Addo Akwetey: resources, formal analysis; data curation. Akosua Bonsu Karikari: formal analysis, writing–review and editing. Simon Kwaku Atta: writing–review and editing, supervision. Mark Michael Addae: conceptualization, supervision.
Ethics Statement
This study was approved by Baldwin University College's ethics and protocol review committee. Before being recruited, all eligible participants provided informed consent and assent.
Conflicts of Interest
The authors declare no conflicts of Interest.
Transparency Statement
The lead author Alahaman Nana Boakye affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
The authors thank the Chief and people of Sempoa for their voluntary participation. We are also grateful to the Staff and management of Ahenfo Medical Centre in Sempoa. We thank all Desk officers of the Neglected Tropical Diseases Control Program, Ghana, and Afrique One ASPIRE for their support.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization , accessed 16 July 2020. https://www.who.int/health-topics/schistosomiasis.
- 2. Fenwick A., Webster J. P., Bosque‐Oliva E., et al., “The Schistosomiasis Control Initiative (SCI): Rationale, Development, and Implementation From 2002–2008,” Parasitology 136, no. 13 (2009): 1719–1730. [DOI] [PubMed] [Google Scholar]
- 3. Ahiadorme M. and Morhe E., “Soil‐Transmitted Helminth Infections in Ghana: A Ten‐Year Review,” Pan African Medical Journal 35 (2020), 10.11604/pamj.2020.35.131.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magalhães R. J., Clements A. C., Patil A. P., Gething P. W., and Brooker S., “The Applications of Model‐Based Geostatistics in Helminth Epidemiology and Control,” Advances in Parasitology 74 (2011): 267–296, 10.1016/B978-0-12-385897-9.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization , Schistosomiasis Progress Report 2001–2011 and Strategic Plan 2012–2020 (Geneva: World Health Organization, 2013). 80. [Google Scholar]
- 6. Ezeamama A. E., Bustinduy A. L., Nkwata A. K., et al., “Cognitive Deficits and Educational Loss in Children With Schistosome Infection: A Systematic Review and Meta‐Analysis,” PLoS Neglected Tropical Diseases 12, no. 1 (2018): e0005524, 10.1371/journal.pntd.0005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohammed E. H., Eltayeb M., and Ibrahim H., “Haematological and Biochemical Morbidity of Schistosoma Haematobium in School Children in Sudan,” Sultan Qaboos University Medical Journal 6, no. 2 (2006): 59–64. [PMC free article] [PubMed] [Google Scholar]
- 8. Bierman W. F. W., Wetsteyn J. C. F. M., and Gool T., “Presentation and Diagnosis of Imported Schistosomiasis: Relevance of Eosinophilia, Microscopy for Ova, and Serology,” Journal of Travel Medicine 12, no. 1 (2005): 9–13, 10.2310/7060.2005.00003. [DOI] [PubMed] [Google Scholar]
- 9. Stanley R. G., Ngaiza J. R., Wambayi E., Lewis J., and Doenhoff M. J., “Platelets as an Innate Defence Mechanism Againstschistosoma Mansoniinfections in Mice,” Parasite Immunology 25, no. 10 (2003): 467–473. [DOI] [PubMed] [Google Scholar]
- 10. Da'dara A. A. and Skelly P. J., “Schistosomes Versus Platelets,” Thrombosis Research 134, no. 6 (2014): 1176–1181, 10.1016/j.thromres.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 11. Joseph M., Auriault C., Capron A., Vorng H., and Viens P., “A New Function for Platelets: IgE‐Dependent Killing of Schistosomes,” Nature 303, no. 5920 (1983): 810–812. [DOI] [PubMed] [Google Scholar]
- 12. Kokaliaris C., Garba A., Matuska M., et al., “Effect of Preventive Chemotherapy With Praziquantel on Schistosomiasis Among School‐Aged Children in Sub‐Saharan Africa: A Spatiotemporal Modelling Study,” Lancet Infectious Diseases 22, no. 1 (2022): 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dejon‐Agobé J. C., Adegnika A. A., and Grobusch M. P., “Haematological Changes in Schistosoma Haematobium Infections in School Children in Gabon,” Infection 49, no. 4 (2021): 645–651, 10.1007/s15010-020-01575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dassah S., Asiamah G. K., Harun V., et al., “Urogenital Schistosomiasis Transmission, Malaria, and Anemia Among School‐Age Children in Northern Ghana,” Heliyon 8, no. 9 (2022): e10440, 10.1016/j.heliyon.2022.e10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savioli L., Hatz C., Dixon H., Kisumku U. M., and Mott K. E., “Control of Morbidity Due to Schistosoma Haematobium on Pemba Island: Egg Excretion and Hematuria As Indicators of Infection,” American Journal of Tropical Medicine and Hygiene 43, no. 3 (1990): 289–295. [DOI] [PubMed] [Google Scholar]
- 16. Hanson C., Weaver A., Zoerhoff K. L., et al., “Integrated Implementation of Programs Targeting Neglected Tropical Diseases Through Preventive Chemotherapy: Identifying Best Practices to Roll Out Pprograms at a National Scale,” American Journal of Tropical Medicine and Hygiene 86, no. 13 (2012): 508–513, 10.4269/ajtmh.2012.11-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linehan M., Hanson C., Weaver A., et al., “Integrated Implementation of Programs Targeting Neglected Tropical Diseases through Preventive Chemotherapy: Proving the Feasibility at a National Scale,” American Journal of Tropical Medicine and Hygiene 84, no. 1 (2011): 5–14, 10.4269/ajtmh.2011.10-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayabina D. V., Clark J., Bayley H., Lamberton P. H. L., Toor J., and Hollingsworth T. D., “Gender‐Related Differences in Prevalence, Intensity and Associated Risk Factors of Schistosoma Infections in Africa: A Systematic Review and Meta‐Analysis,” PLoS Neglected Tropical Diseases 15, no. 11 (2021): e0009083, 10.1371/journal.pntd.0009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ismail H. A., Hong S. T., Babiker A. T., et al., “Prevalence, Risk Factors, and Clinical Manifestations of Schistosomiasis Among School Children in the White Nile River Basin, Sudan,” Parasites & Vectors 7 (2014): 478, 10.1186/s13071-014-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angelo T., Kinung'hi S. M., Buza J., Mwanga J. R., Kariuki H. C., and Wilson S., “Community Knowledge, Perceptions, and Water Contact Practices Associated With Transmission of Urinary Schistosomiasis in an Endemic Region: A Qualitative Cross‐Sectional Study,” BMC Public Health 19, no. 1 (2019): 703, 10.1186/s12889-019-7041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Afrifa J., Gyedu D., Ofori Gyamerah E., Essien‐Baidoo S., and Mensah‐Essilfie I., “Haematological Profile and Intensity of Urogenital Schistosomiasis in Ghanaian Children,” Journal of Environmental and Public Health 2017 (2017): 4248325, 10.1155/2017/4248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pivina L., Semenova Y., Doşa M. D., Dauletyarova M., and Bjørklund G., “Iron Deficiency, Cognitive Functions, and Neurobehavioral Disorders in Children,” Journal of Molecular Neuroscience 68 (2019): 1–10, 10.1007/s12031-019-01276-1. [DOI] [PubMed] [Google Scholar]
- 23. Rujeni N., Taylor D. W., and Mutapi F., “Human Schistosome Infection and Allergic Sensitisation,” Journal of Parasitology Research 2012 (2012): 154743, 10.1155/2012/154743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ugbomoiko U. S., Dalumo V., Ariza L., Bezerra F. S. M., and Heukelbach J., “A Simple Approach Improving the Performance of Urine Reagent Strips for Rapid Diagnosis of Urinary Schistosomiasis in Nigerian Schoolchildren,” Memórias do Instituto Oswaldo Cruz 104, no. 3 (2009): 456–461, 10.1590/s0074-02762009000300010. [DOI] [PubMed] [Google Scholar]
- 25. Bogoch I. I., Andrews J. R., Dadzie Ephraim R. K., and Utzinger J., “Simple Questionnaire and Urine Reagent Strips Compared to Microscopy for the Diagnosis of Schistosoma Haematobium in a Community in Northern Ghana,” Tropical medicine & international health: TM & IH 17, no. 10 (2012): 1217–1221. [DOI] [PubMed] [Google Scholar]
- 26. Kosinski K. C., Bosompem K. M., Stadecker M. J., et al., “Diagnostic Accuracy of Urine Filtration and Dipstick Tests for Schistosoma Haematobium Infection in a Lightly Infected Population of Ghanaian Schoolchildren,” Acta Tropica 118, no. 2 (2011): 123–127, 10.1016/j.actatropica.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27. Kotb M. M., Shouman A. E., Hussein H. M., Khela A. K., and Kandil S. K., “Evaluation of the Effectiveness of Dipstick Haematuria and Proteinuria in Screening Schistosoma Haematobium Infection Among School Children in Upper Egypt,” Journal of the Egyptian Public Health Association 71, no. 5–6 (1996): 353–367. [PubMed] [Google Scholar]
- 28. Simerville J. A., Maxted W. C., and Pahira J. J., “Urinalysis: A Comprehensive Review,” American Family Physician 71, no. 6 (2005): 1153–1162. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


