Abstract
Hydrogen-transfer is the primary process responsible for elevating the degree of unsaturation of intermediates in zeolite-catalyzed methanol-to-hydrocarbon reactions, with olefins serving as the typical receptor and alkanes being produced as the by-product. Intriguingly, the introduction of CO was shown to suppress the selectivity of alkanes and enhance the production of aromatics, yet microscopic understanding of this phenomenon remains elusive. Here, based on ab initio molecular dynamics simulations and free energy sampling methods, we discover a non-olefin-induced hydrogen-transfer reaction in the presence of CO, with ketene/acetyl emerging as a more suitable hydrogen-transfer receptor than olefins. This predominant route enhances the degree of unsaturation of olefins without generating additional alkanes, and the produced dienes and acetaldehyde could further contribute to the formation of aromatics. Moreover, we construct a general mechanism applicable to a series of CO-coupled aromatics synthesis reactions, offering distinctive insights and strategies for the optimization of efficiency.
Subject terms: Molecular dynamics, Catalytic mechanisms, Heterogeneous catalysis
The role of CO in zeolite-catalyzed methanol-to-hydrocarbon reactions remains unclear. Here, ab initio molecular dynamics and free energy sampling reveal a non-olefin-induced hydrogen transfer mediated by CO, with ketene/acetyl identified as a superior hydrogen-transfer receptor over olefins.
Introduction
Aromatics are fundamental chemical raw materials that play a crucial role in the production of various commodities, including polymers, dyes, perfumery, medicines, and synthetic rubbers. The consumption of aromatics has been pivotal in shaping modern life and continues to be in high demand1. Conventionally, the production of aromatics relied heavily on the petroleum route, which primarily involves naphtha reforming and steam cracking. However, with the depletion of crude oil reserves and the environmental concerns associated with traditional synthetic methods, there is a growing interest in developing sustainable production technologies from non-petroleum carbon sources such as natural gas, coal, and biomass. As such, extensive research efforts are being directed towards this end.
In contemporary times, natural gas has emerged as a pivotal and environmentally-friendly non-petroleum resource for producing aromatics2,3. The direct synthesis of aromatics from methane, without the involvement of other agents, is a highly economical and sustainable approach. However, this method poses a challenge due to the inert nature of methane, which limits its reactivity4–6. Alternatively, methane can be transformed into agents with higher activity, such as syngas, methanol, and methyl halide, thereby offering a viable compromise. Although these indirect methods are still in the nascent stages of laboratory-scale experimentation, their potential impact is significant and warrants further research1,7–13.
In the quest to convert these intermediates into aromatics, zeolite, a prototypical porous material, has emerged as an ideal catalyst. During these transformations, zeolite fulfills two primary functions. Firstly, it provides Brønsted acid sites (BASs) that can activate species typically considered inert14–16. Secondly, the atoms surrounding its inner pores can elicit van der Waals interactions onto the molecules. This interaction is critical in zeolite catalysis and has a significant impact on the selectivity and transport properties of the overall reaction17,18.
Regarding the typical processes for the synthesis of aromatics, such as the conversion of CH3Cl (methyl chloride) to aromatics (CTA), and CH3OH (methanol) to aromatics (MTA), with zeolite as a catalyst, only low selectivity for aromatics (<50%) could be obtained due to the unavoidable hydrogen-transfer reaction between olefins, leading to the formation of alkanes as by-products. A typical mechanism of hydrogen-transfer is presented in Fig. 1a. However, such hydrogen-transfer reactions are essential for the production of highly unsaturated hydrocarbon molecules19–22. Recently, several studies have made significant strides in enhancing the selectivity of aromatics through a novel strategy involving the participation of CO23,24. For example, in the CCTA process, the introduction of CO effectively reduces the selectivity for C2–C4 paraffins to 3.1%, while the selectivity for aromatics is to as high as 82.2%. A common observation is that following the participation of CO, the selectivity of aromatics is markedly increased, while the selectivity of alkanes remains low, indicating that the olefin-induced hydrogen-transfer (OHT) reactions is attenuated and there must be a new type of hydrogen-transfer processes running in the system. However, the precise mechanism underlying this transformation remains elusive.
Fig. 1. Mechanism of hydrogen-transfer reaction.
a Typical reaction mechanism of hydrogen-transfer reaction. Notations in the brackets represent the difference of hydrogen atom numbers from the original molecules. b Olefin-induced hydrogen-transfer (OHT) route with ethylene and butylene being the receptor and donor, respectively. c Ketene-induced hydrogen-transfer (KHT) route with ketene and butylene being the receptor and donor, respectively. Oz represent the oxygen atoms of BAS. The orange hydrogen represented the hydride ion that transfers between hydride-donor and receptor.
An intriguing observation in this reaction is the significant presence of surface acetyl groups or related species during the initial stages of the reaction, particularly in the presence of CO23,24. In fact, the deprotonation of surface acetyl groups is postulated to result in the formation of ketene, which has conventionally been regarded as a key product in the formation of the first C-C bond in methanol-to-hydrocarbon processes25,26. The existence of ketene has been confirmed both experimentally and theoretically in various catalytic systems27–29. Moreover, the detection of oxygenates, such as acetone, as reaction intermediates suggests that further transformations of the surface acetyl group may occur30,31, potentially leading to the formation of oxygenates with lower saturation levels.
Based on above evidences, existence of a more advantageous reaction pathway than the OHT pathway would shed light on the underlying reason for higher aromatics selectivity and lower alkane selectivity. It can be inferred that under these reaction conditions, ketene/acetyl may serve as a viable candidate for the role of a hydrogen-transfer receptor. In other words, hydrogen-transfer reactions are likely to occur preferentially between the ketene-related species and olefin, rather than between two olefins through OHT. This postulation has the potential to concurrently address the following three issues: (i) the reason behind the enhanced selectivity of aromatics with the addition of CO, (ii) the suppression of alkane selectivity, or equivalently, OHT reactions under these conditions, and (iii) the function and fate of surface acetyl group-related species in the early stages of the reaction.
In this study, we employed ab initio molecular dynamics (AIMD) simulations with enhanced free energy sampling, i.e., metadynamics (MTD), to investigate the possible mechanisms in detail. The MTD method has been adopted due to its ability to explore complex and high dimensional free energy surfaces32,33. Our investigation commenced with a consideration of the corresponding rival hydride receptors, i.e., ethylene and ketene/acetyl34. Butylene was included as another reactant in this model reaction, acting as a hydride donor, which is commonly used in related research. We then constructed a network of OHT and ketene-induced hydrogen-transfer (KHT) reactions and simulated each elementary reaction. The free energy was obtained to compare the thermodynamic and kinetic properties between the two rival routes. Furthermore, we conducted structural analyses to elucidate the detailed evolution of the system along the reaction coordinate. Ultimately, the findings confirmed our prior assumptions and provided a plausible general mechanism for the aforementioned CO-participated processes.
Results
Reaction Scheme
The hydrogen-transfer reaction plays a pivotal role in aromatics synthesis, which follows a dual-cycle mechanism35–38. The species involved significantly influence the free energy barrier of hydrogen-transfer reaction, and thus the selectivity and reactivity of the overall reaction. Prior studies have suggested that hydrogen-transfer can take place either between two alkenes or between an alkene and methanol. However, the energy barrier associated with methanol-induced hydrogen-transfer is comparatively high, which allows us to consider this pathway less significant22. Figure 1a illustrates a typical hydrogen-transfer reaction, which comprises three elementary steps19–21. Initially, the proton at the BAS binds to the hydride receptor, generating a cation. Subsequently, the hydride donor donates a hydride ion to this cation, resulting in a hydride receptor with a lower degree of unsaturation. Finally, the reaction concludes with the deprotonation of the hydride donor, producing a species with a higher degree of unsaturation.
Based on the assumption mentioned in the Introduction section, we selected ethylene and ketene as suitable candidates for the hydride receptor in a series of competing reactions. We will show later that considering more complex hydride receptors in the OHT process, such as propylene and butylene, will not alter the conclusions made. Butylene was chosen as the most appropriate species to act as a hydride donor, given that it is the simplest molecule capable of forming a diene19. In fact, previous study found that the structure of hydride donor would show marginal effect on the activation free energy38. The entire reaction is considered to involve stepwise protonation and the transfer of hydride ion, since previous research has shown that a concerted mechanism possesses a higher energy barrier22. The reaction mechanisms for both OHT and KHT routes are presented in Fig. 1b, c, and will be further elaborated on in subsequent sections.
Protonation of the hydride receptor
Figure 2 presents the free energy surface (FES) of the protonation of ethylene (REt,1). Two collective variables (CVs), the relevant concepts and aims of which can be referred to the “Methods” section, are used here. CV1 (x-axis) represents the transfer of the proton between BAS and ethylene, while CV2 (y-axis) describes the adsorption/desorption of ethylene. There are three important regions in this FES, namely the initial state (IS), transition state (TS), and final state (FS). The bottom-right deep basin corresponds to the IS region, representing the configuration of ethylene and the adsorbed proton. The upper-left deep basin corresponds to the FS region, representing the configuration of the surface ethoxy species. The bottom-left saddle represents the TS region and contains critical kinetic information about this reaction, which will be discussed below. The free energy barrier of this process is relatively high, which is 91.68( ± 1.76) kJ mol−1, close to previous DFT results (83 kJ mol−1)22. The reaction free energy of this reaction is 9.32( ± 1.62) kJ mol−1, indicating that the surface ethoxy is a relatively unfavored species.
Fig. 2. FES of protonation of ethylene (REt,1) and the representative configurations.
IS, TS and FS represent the initial state, transition state and final state, respectively. Gray, white, red and violet balls represent carbon, hydrogen, oxygen and aluminum atoms respectively. The red and orange lines represent the residual silica skeleton. The depth of the color bar represents the relative free energy.
Figure 3 illustrates the FES of the protonation of ketene (RKt,1). In this graph, both CV1 and CV2 represent the transfer of proton from BAS to ketene. Similar to the FES of ethylene protonation, there are three crucial regions in this FES. The FS is a depth basin at the bottom of this graph, representing the configuration of an acylium. The IS and TS are somewhat indistinct due to the relatively low activation free energy of this reaction. The free energy barrier is only 8.96( ± 1.78) kJ mol−1, and the reaction free energy is -23.35( ± 3.34) kJ mol−1. These results exhibit similar characteristics to MTD studies in MOR and SSZ-13, where acylium is a thermodynamically favored species over ketene26. Nevertheless, it is worth noting that the different topology of zeolite can have a non-negligible impact on the distribution of ketene.
Fig. 3. FES of protonation of ketene (RKt,1) and the representative configurations.
IS, TS and FS represent the initial state, transition state and final state, respectively. Gray, white, red and violet balls represent carbon, hydrogen, oxygen and aluminum atoms respectively. The red and orange lines represent the residual silica skeleton. The depth of the color bar represents the relative free energy.
One may notice that the selection of CVs differs in the above two reactions. In fact, to obtain reliable results from MTD simulations, the CVs and walls that exclude the side reactions must be carefully designed based on the characteristics of reaction. In these simulations, the FS required further analysis of the adsorption process since there can be several possible existence forms of the product. In detail, the FS of protonation of ethylene could be surface ethoxy species or ethyl cation, and the FS of protonation of ketene could be surface acetyl group or acylium15,16. Although several DFT studies have investigated these two adsorption processes, there is a lack of simulations in H-ZSM-5 zeolite at high temperatures22,26. Therefore, we conducted detailed simulations to strengthen the subsequent research and provide valuable insights into the mechanism of these reactions.
Two 1D FES graphs corresponding to the desorption of surface ethoxy species and surface acetyl group are presented in Supplementary Figs. 1 and 2. It is evident that the desorption of the surface ethoxy species is quite difficult, with a free energy barrier of up to 85.92( ± 3.13) kJ mol−1. Therefore, we have selected the surface ethoxy species as the FS, and the CV related to adsorption must be included in our analysis. In contrast, the desorption of the surface acetyl group exhibits a significantly lower barrier, with only a negligible free energy barrier of 15.96( ± 1.80) kJ mol−1. Since the metadynamics simulation of a simpler process often leads to a better convergent result, we here chose acylium as the FS in the simulations.
The details of both reactions can be understood by analyzing the evolution of critical structural variables. For the protonation of ethylene, we analyzed the evolution of the absolute value of the difference between the proton’s distance to the two carbon atoms (absDiffEt) along the reaction coordinate, and the result is shown in Fig. 4. One can find that, before the system reaches the TS region, absDiffEt remains a small value, indicating that ethylene has no apparent orientation towards proton. However, this situation changes rapidly near the TS, where absDiffEt experiences a quick surge, and the proton attaches to one carbon atom of ethylene, corresponding to the formation of an adsorbed surface ethoxy species. For the protonation of ketene, we analyzed the evolution of a similar variable, namely the difference between the distances of the proton to the terminal carbon atoms and that to the central carbon (DiffKt). Interestingly, this value exhibits a steady decrement during this reaction and stays negative, indicating that the proton always reacts with the terminal carbon, and the polarity of ketene does not shift significantly.
Fig. 4. Evolution of the structural parameters and free energy along the reaction coordinates.

a The absolute value of the difference between the proton’s distance to the two carbon atoms of ethylene (absDiffEt) along the reaction coordinate of protonation of ethylene. b The difference between distances from proton to the terminal carbon atoms and that to the central carbon (DiffKt) along the reaction coordinate of protonation of ketene. Red and violet lines represent the chosen data and the free energy respectively. The pink shadow represents the uncertainty of the chosen data.
Transfer of hydride ion between the donor and receptor
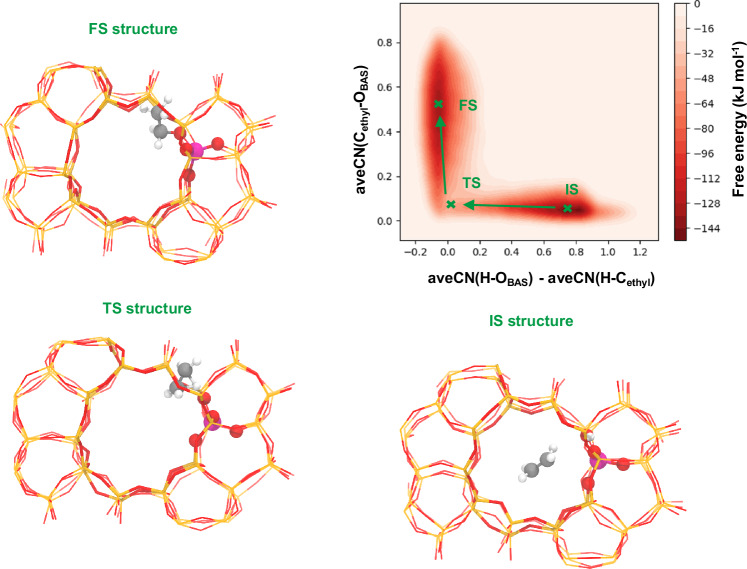
Figure 5 shows the FES of transferring a hydride ion between surface ethoxy species and butylene (REt,2). CV1 (x-axis) describes the transfer of the hydride ion between butylene and the surface ethoxy species, and CV2 (y-axis) describes the adsorption/desorption process of the surface ethoxy species. The constituents of this graph are relatively intricate. In addition to the IS, FS, and TS regions, a metastable state (MS) region also exists, implying that this process can be a competition between a concerted reaction mechanism and a stepwise one. This is rather interesting because both mechanisms can be identified from the FES. The upper-left basin corresponds to the IS region, representing a surface ethoxy species and a butylene, while the FS region is the bottom-right basin, representing ethane and a protonated butadiene. As a digression, the state of protonated butadiene, which is desorbed in this FS, is consistent with previous research for its significant steric hindrance at BAS16,38. The marked TS region is a saddle point whose structure is subtle and discussed at length in the following paragraph.
Fig. 5. FES of transfer of hydride ion between surface ethoxy species and butylene (REt,2) and the representative configurations.
IS, TS, MS and FS represent the initial state, transition state, metastable state and final state, respectively. Gray, white, red and violet balls represent carbon, hydrogen, oxygen and aluminum atoms respectively. The red and orange lines represent the residual silica skeleton. The depth of the color bar represents the relative free energy.
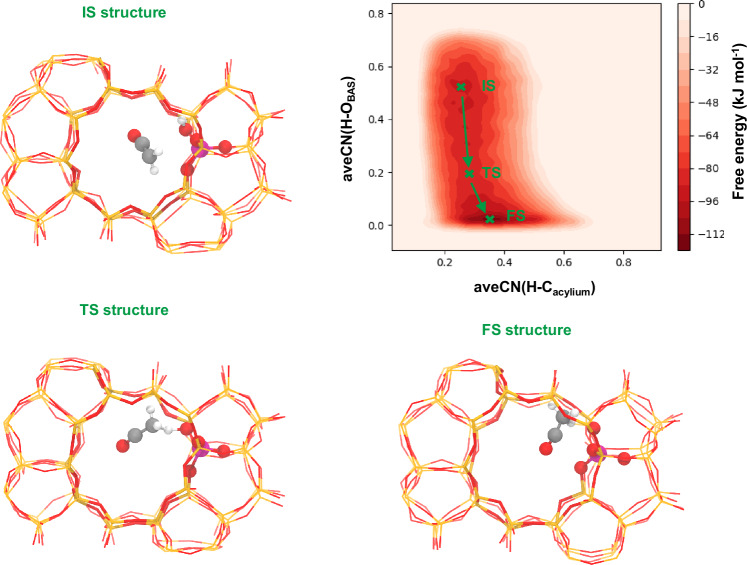
Figure 6 presents the FES of transferring a hydride ion between acylium and butylene (RKt,2). The x and y-axes describe the length of the bond between the hydride ion and butylene and acetyl, respectively. Since this reaction is unrelated to the desorption/adsorption process, its FES is much simpler than that of hydride ion transfer between the surface ethoxy species and butylene. The bottom-right basin represents the IS region, corresponding to an acylium and butylene, while the upper-left basin represents the FS area, consisting of a desorbed protonated butadiene and an acetaldehyde. The marked saddle point corresponds to the TS region. It should be apparent from the representing structure that the hydride ion attaches to both the acetyl cation and butylene. Further details regarding the kinetic information will be discussed below.
Fig. 6. FES of transfer of hydride ion between acylium and butylene (RKt,2) and the representative configurations.
IS, TS and FS represent the initial state, transition state and final state, respectively. Gray, white, red and violet balls represent carbon, hydrogen, oxygen and aluminum atoms respectively. The red and orange lines represent the residual silica skeleton. The depth of the color bar represents the relative free energy.
To obtain a detailed understanding of the kinetic features of the reactions, we analyzed the evolution of several vital variables along the reaction coordinates in detail. There are two important sets of variables that can describe these reactions quite well. The first set of variables pertains to the evolution of the relative positions among surface ethoxy species/acylium, butylene, and the center of BAS, which we denoted as C2, C4, and Al, respectively. The second set of variables describes the transfer of the hydride ion between C2 and C4.
The relative position of reactants during the transfer of hydride ion between the surface ethoxy species and butylene are presented in Fig. 7b–d. In Fig. 7b, we observe that the distance between surface ethoxy group and butylene initially decreases and then increases, indicating that the hydride-transfer reaction requires the two reactants to be in close proximity. Interestingly, the intramolecular distance reaches its minimum not at the transition state but rather near the final stage of this reaction. The implications of this result should be explored with the aid of the second set of variables, which will be discussed later. In Fig. 7c, we observe that the ethyl cation moves away from the BAS, corresponding to the desorption of the surface ethoxy species and the formation of ethane. The trends in the relative distance between butylene and the center of BAS are shown in Fig. 7d. It initially increases before reaching the transition state, as the desorption of surface ethoxy species imposes an additional repulsive force on butylene. After the transition state, it decreases due to the attractive interaction between the zeolite skeleton with a negative charge and the protonated butadiene with a positive charge.
Fig. 7. Evolution of the structural parameters and free energy along the reaction coordinates of transfer of hydride ion between surface ethoxy species and butylene (REt,2).
a The scheme for physical meaning of the chosen data. Hydride ion, surface ethoxy species, butylene and the center of BAS are denoted as H, C2, C4 and Al, respectively. Distance between species a and b are denoted as D(a-b). Oz represent the oxygen atoms of BAS. b–f Red and violet lines represent the chosen data and the free energy respectively. The pink shadow represents the uncertainty of the chosen data.
Transfer of the hydride ion between the surface ethoxy species and butylene is presented in Fig. 7e, f, which depict the evolution of the distance between butylene/surface ethoxy species and the hydride ions. We can clearly observe that the bond between butylene and the hydride ion does not break until the final stage of the whole reaction, while the distance between the hydride ion and surface ethoxy species begins to decrease at the initial stage of this reaction. By combining the trend of the intramolecular distance between surface ethoxy species and butylene, we can deduce that the hydride ion transfers from butylene to the surface ethoxy species in the final stage of this reaction.
The relative positions of the reactants during the transfer of the hydride ion between acylium and butylene are presented in Fig. 8b–d. Similar to the results of the ethyl-butylene hydride-transfer discussed above, the distance between acylium and butylene, as shown in Fig. 8b, initially decreases and then increases, with the overall minimum near the transition state region. In Fig. 8c, d, we observe that the distance between the reactants and aluminum atoms (D(C2-Al)/D(C4-Al)) evolves monotonically. According to Supplementary Fig. 3, the value of (D(C2-Al) - D(C4-Al)) increases from a negative value to a positive one, representing a swapping of the relative position of both species caused by the charge transfer between hydride donor and acceptor.
Fig. 8. Evolution of the structural parameters and free energy along the reaction coordinates of transfer of hydride ion between acylium and butylene (RKt,2).
a The scheme for physical meaning of the chosen data. Hydride ion, acylium, butylene and the center of BAS are denoted as H, C2, C4 and Al, respectively. Distance from species a to b is denoted as D(a-b). Oz represent the oxygen atoms of BAS. b–f Red and violet lines represent the chosen data and the free energy respectively. The pink shadow represents the uncertainty of the chosen data.
The evolution of the hydride ion during the transfer between acylium and butylene is presented in Fig. 8e, f. In contrast to the reaction involving the surface ethoxy species, we observe both breaking of the bond between butylene and the hydride ion and formation of the bond between acylium and the hydride ion occurs near the transition state simultaneously.
Upon analyzing the dynamic features of these two reactions, we can infer that the free energy barrier for the transfer of the hydride ion primarily arises from two factors. The first factor is the requirement for the two reactants to be in close proximity, which introduces repulsive steric hindrance during this process. The second factor is the stability of the hydride receptor in forming a cation. Considering these two reactions, it is evident that acetyl is more prone to desorption from BAS and can readily form a stable cation, resulting in a lower overall free energy barrier for the reaction involving acetyl.
Free energy alignment and comparison of hydrogen-transfer routes
In this study, we conducted a detailed analysis of the key elementary steps of the two competing OHT and KHT routes separately. However, a critical question arises regarding how to compare these two routes with an aligned free energy reference. This question is equivalent to construct a reaction pathway to connect one intermediate in each route and determine the free energies under the reaction conditions. In this way, the relative abundance of the reactants for the two routes can be further related. The choice of this transformation route has a profound influence on our conclusions because the free energy difference between these two intermediates may depend not only on themselves but also on other agents involved in this transformation pathway.
Previous studies on the mechanism have revealed that surface acetyl group could further deprotonate and form a ketene, which could diffuse to another reactive site. Then ketene could also undergo methylation and decarbonylation successively, as one can see from Fig. 9a34,35. Specifically, a surface methoxy species can react with ketene to form a propionyl cation, which then discards a carbon monoxide molecule, leaving behind a surface ethoxy species. Therefore, it is reasonable to select methylation of ketene (Ralign,1) and decarbonylation of propionyl cation (Ralign,2) as the elementary reactions of this transformation23.
Fig. 9. Free energy alignment of overall reaction route.
a Reaction route of the transformation including methylation of ketene (Ralign,1) and decarbonylation of propionyl cation (Ralign,2). b Free energy results of the transformation. c Structures of transition states of the transformation. d free energy results of overall reaction route. Red, green and gray component represent the OHT route, KHT route and the transformation, respectively. Two states of acetyl species are included here which can reach a rapid equilibrium, (ads) and (des) represent the surface acetyl group and acylium, respectively.
The FES for both reactions are presented in Supplementary Figs. 4 and 5. The whole reaction free energy is the most critical parameter of interest, which is −7.93 kJ mol−1 from ketene to surface ethoxy species. The free energy results and structures of transition states are presented in Fig. 9b, c. By using the reaction between the reactants of both routes, we can connect them, as shown in Fig. 9d. The deprotonation of protonated butadiene (REt,3 and RKt,3) was also included in this scheme, and the detailed results are shown in Supplementary Fig. 6. However, its low free energy barrier has a negligible influence on our conclusion.
After considering the alignment of the two routes, we can conclude that the rate-determining transition state of the KHT route is the transfer of the hydride ion, while both the adsorption and transfer of the hydride ion in the OHT route are confronted with high free energy barriers. Therefore, we can conclude that the hydrogen-transfer between ketene and butylene is a more advantageous route when taking the transformation between ketene and ethylene into consideration. More importantly, we further considered two additional possible OHT routes involving hydrogen-transfer to form propane and butane, the results of which are presented and discussed in Supplementary Note 1. It should be mentioned that the MTD sampling method was initially used for these simulations, but several problems were encountered. Therefore, we have conducted four sets of slow-growth simulations for the key elementary hydride transfer step of these four routes (i.e., hydrogen-transfer between ketene/C2/C3/C4 and C4), and ten parallel simulations were performed for each route. The overall results indicate that the OHT pathways all have higher activation free energies, indicating that the KHT pathway is still dominant.
Reaction mechanism of the early stage of CO-coupled aromatics synthesis
Based on the above analyzes, it is possible to construct a general mechanism to describe the early stage of aromatics synthesis in the presence of CO, e.g., CO-coupled CTA (CCTA) and CO-coupled MTA (CMTA). The proposed mechanism is shown in Fig. 10. In the initial stage, surface methoxy species is easily formed at BAS and transforms into a surface acetyl group with the help of CO. After a series of methylation and decarbonylation reactions, various alkenes are produced26,34,39,40. Following previous researches, it is generally recognized that alkanes predominantly originate from hydrogen-transfer reactions, hydrogenation processes41, and alkane cracking22,42. However, it is primarily the hydrogen-transfer that significantly influences the selectivity of alkanes. Traditionally, hydrogen-transfer occurs between alkenes, and the saturation of some alkenes arises at the cost of producing alkanes as by-products. However, as explained in the above paragraphs, ketene or the surface acetyl group is a more suitable hydride receptor in the early stage due to the much lower free energy barrier this process gives.
Fig. 10. Proposed general mechanism for the early stage of CO-coupled CH3X to aromatics, X = OH, OCH3, Cl, etc.

The olivaceous bold part shows the reaction related to our proposed mechanism. The black plain part is the traditional mechanism in previous studies.
It is noteworthy to highlight that the predominant KHT process in the reaction system results in a reduction in the selectivity of alkanes. The diene can readily react with other alkenes to form a cycloalkene, which subsequently undergoes transformation into aromatics43. Acetaldehyde, the other product of the hydrogen-transfer reaction, was also detected in the methanol-to-olefin process and considered to be highly reactive28. Experiments utilizing isotopically labeled 13CO indicates that 2,3-dimethyl-2-cyclopentene-1-one (DMCPO)23,24,44, an important intermediate in CCTA and CMTA, contains at least one 13C atom. Approximately 60% to 70% of the DMCPO analyzed possess a 13C atom, suggesting that these 13C-labeled species may have originated from the cyclization of polyenes produced via KHT in conjunction with acetaldehyde. The generation of DMCPO containing multiple 13C atoms can be attributed to the Prins-dehydroacylation mechanism described in the literature1,7,8,24,45. This implies that aldehydes can participate in the Prins reaction with various olefins, facilitating the formation of dienes46. Through a series of steps involving hydroacylation and aldol reactions with multiple aldehydes, DMCPOs are ultimately produced. Finally, the products of both pathways, cycloalkane and DMCPO, can undergo transformation into aromatics, following mechanisms proposed in the previous study47. One can find that this KHT reaction plays a pivotal role as a nexus between several established mechanisms. These findings also suggest that CO or CO-related reactants play a crucial role in driving the unsaturation of the hydrocarbon pool.
The presence of ketene/acetyl has also been observed in an oxide-zeolite-catalyzed syngas-to-aromatics (STA) reaction29,48,49. In this scenario, CO is only partially consumed in the entire STA reaction, achieving a conversion of approximately 20%. This result implies that the amount of CO is abundant within the zeolite, similar to CO-coupled reactions such as CCTA and CMTA. Consequently, evidence of the association between CO and ketene/acetyl can be discerned from an alternative perspective. Collectively, these findings furnish evidence of the potential role of ketene/acetyl in this series of analogous reactions and provide insights into their optimization.
Discussion
In this work, we gained a comprehensive understanding of the hydrogen-transfer reaction in CO-coupled aromatics synthesis in zeolites. Through metadynamics simulations, we compared the competition between the two routes of the hydrogen-transfer reaction and found that the ketene serves as a more suitable hydride receptor than ethylene in the early stage, resulting in a lower free energy barrier for this process. This predominant route increases the degree of unsaturation of olefins without producing additional alkanes. Furthermore, the pivotal role of produced dienes and acetaldehyde from the KHT process as critical intermediates in CCTA and CMTA has been underscored. These findings lead to the construction of a general mechanism describing the early stage of aromatics synthesis in the presence of CO, such as CCTA and CMTA. Additionally, evidence of the association between CO and ketene/acetyl in oxide-zeolite-catalyzed STA reactions has been discussed, suggesting the potential role of ketene/acetyl in these analogous reactions and providing insights into their optimization. Overall, these findings contribute to a deeper understanding of the mechanisms underlying CO-coupled aromatics synthesis and highlight the importance of ketene/acetyl in these processes.
Methods
AIMD simulation
All AIMD simulations were performed using the CP2K software package50. In the simulations, we selected a constant particle number, volume, and temperature (NVT) ensemble. The temperature was set to 673 K to ensure consistency with experimental conditions23. To simulate electron behavior, we employed Gaussian plane-wave basis sets (GAW) and the revPBE functional. We opted to replace traditional functionals with revPBE due to its superior ability to describe energies related to catalytic processes in solid-state calculations compared to commonly used functionals, such as PBE51,52. The Goedecker-Teter-Hutter (GTH) norm-conserving pseudopotentials were used to describe the core electrons, and the shorter-range molecularly optimized GTH basis sets with an energy cutoff of 280 Ry were chosen to expand electronic wavefunctions53. Given that van der Waals interactions play a critical role in zeolite, we utilized the DFT-D3 method to better describe dispersive interactions54,55. To obtain reliable behavior of hydrogen atoms, we employed a time-step of 0.5 fs in the MD simulations, and the mass of the hydrogen atom was chosen to be 2 a.m.u. to keep the stability of the structure of hydrogen-containing species56–58. Additionally, we used the Nosé-Hoover thermostat with three chains to maintain the temperature at 673 K59.
Free energy sampling
All free energy samplings were performed using the PLUMED plugin. In our simulations, we employed metadynamics (MTD) to simulate the FES at high temperatures60–62. MTD is a biasing enhanced sampling technique that exerts an artificial biasing potential towards the physical FES. A typical MTD requires two important prior settings. The first setting is the collective variable (CV), which can be any variable that describes the evolution of the reaction. In this study, we selected combinations of coordination numbers (CNs) as the CV:
| 1 |
where rij is the distance between atoms i and j, and nn and nd were set to 6 and 12, respectively. Previous research has shown that CN is a suitable CV for describing the evolution of typical chemical reactions, which often involve bond formation and breaking62–64. Detailed information regarding the CV settings for each reaction is listed in Supplementary Table 2.
The second setting involves determining how to exert the biasing potential. In this simulation, we employed a Gaussian biasing potential with a width of 0.03 throughout the entire simulation. Its height was initially set to 2.6255 kJ mol−1 (0.001 Hartree) and then reduced to 1.3128, 0.6564, and 0.3282 kJ mol−1 to improve the accuracy of the results. The interval between exerting a bias was 25 fs (50 steps) for two-dimensional (2D) MTD and 50 fs (100 steps) for one-dimensional (1D) MTD, respectively, to ensure that the system had enough relaxation before FES changes. Moreover, quadratic walls were used to prevent possible side-reactions, and detailed information regarding these settings is listed in Supplementary Table 3. The evolution of CV1-CV2 and the moments to reduce the height of Gaussian hills are presented in Supplementary Fig. 7.
For 2D MTD simulations, we employed a projection method to obtain the free energy profile. We selected the difference between CVs, i.e., CV1 – CV2, as the reaction coordinate and projected the 2D FES onto this 1D coordinate. This projection method was introduced by Moors et al.62,65,66, and is expressed by the following equation:
| 2 |
After this projection procedure, we could obtain activation free energy and reaction free energy. All the free energy results are shown in Supplementary Table 1.
To analyze the structure evolution along reaction coordinate, we employed a software package named MULE to find the most probable reaction route among the reaction path ensemble in the grids of FES67. We carefully chose small vicinities of grids (± 0.01) on the FES of the reaction route and used the corresponding configurations for structural analysis. These statistical settings were selected to ensure the accuracy and reliability of our results.
Catalyst model
The catalyst H-ZSM-5 was modeled in a periodic orthorhombic cell obtained from the Database of Zeolite Structure website68. We conducted a 50 ps simulation using the NPT ensemble, where the temperature and pressure were set to be consistent with the reaction conditions, specifically 673 K and 1 bar. The initial 10 ps of the simulation was dedicated to relaxation, and the average was calculated over the remaining time. The actual dimensions of the zeolite cell were (a, b, c) = (20.159, 20.035, 13.474) Å, (α, β, γ) = (90, 90, 90)°. To create a BAS, we select the T9 to be a suitable site, which is easy to be replaced by aluminum according to previous research69. This site is located at the channel intersection, which was identified as an active environment in related research65,70. The central aluminum atom is connected to four oxygen atoms, each of which can participate in reactions with reactants. Therefore, it is reasonable to assign equal importance to these oxygen atoms in the MTD simulations.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22322302, 22072091, 92045301) and National Key Research and Development Program of China (2022YFA1503804). We thank the HPC Platform of ShanghaiTech University and Shanghai Supercomputer Center for computing time.
Author contributions
Z.G. performed the AIMD simulations. Q.C. and J.L. performed further analyzes based on AIMD simulations. B.Y. conceived the problem. All the authors contributed to writing the paper.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data generated in this study are provided in the Supplementary Information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-55514-1.
References
- 1.Li, T., Shoinkhorova, T., Gascon, J. & Ruiz-Martínez, J. Aromatics production via methanol-mediated transformation routes. ACS Catal.11, 7780–7819 (2021). [Google Scholar]
- 2.Olah, G. A. Beyond oil and gas: the methanol economy. Angew. Chem. Int. Ed.44, 2636–2639 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science367, 193–197 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev.117, 8497–8520 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kosinov, N., Coumans, F. J., Uslamin, E., Kapteijn, F. & Hensen, E. J. Selective coke combustion by oxygen pulsing during Mo/ZSM-5-catalyzed methane dehydroaromatization. Angew. Chem., Int. Ed. Engl.55, 15086–15090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, X., Pei, C. & Gong, J. Shale gas revolution: catalytic conversion of C1–C3 light alkanes to value-added chemicals. Chem7, 1755–1801 (2021). [Google Scholar]
- 7.Ni, Y., Zhu, W. & Liu, Z. H-ZSM-5-catalyzed hydroacylation involved in the coupling of methanol and formaldehyde to aromatics. ACS Catal.9, 11398–11403 (2019). [Google Scholar]
- 8.Ni, Y., Zhu, W. & Liu, Z. Formaldehyde intermediate participating in the conversion of methanol to aromatics over zinc modified H-ZSM-5. J. Energy Chem.54, 174–178 (2021). [Google Scholar]
- 9.Gamero, M. et al. Kinetic model for the conversion of chloromethane into hydrocarbons over a hzsm-5 zeolite catalyst. Ind. Eng. Chem. Res.57, 908–919 (2018). [Google Scholar]
- 10.Gamero, M., Valle, B., Castaño, P., Aguayo, A. T. & Bilbao, J. Reaction network of the chloromethane conversion into light olefins using a HZSM-5 zeolite catalyst. J. Ind. Eng. Chem.61, 427–436 (2018). [Google Scholar]
- 11.Valecillos, J., Manzano, H., Aguayo, A. T., Bilbao, J. & Castaño, P. Kinetic and deactivation differences among methanol, dimethyl ether and chloromethane as stock for hydrocarbons. ChemCatChem11, 5444–5456 (2019). [Google Scholar]
- 12.Kwon, S. et al. CH4 chlorination with Cl2 using zeolites having different surface polarities: catalysis descriptors explaining the electrophilic pathway. J. CO2 Util. 42, 10.1016/j.jcou.2020.101318 (2020).
- 13.Bilke, M., Losch, P., Vozniuk, O., Bodach, A. & Schuth, F. Methane to chloromethane by mechanochemical activation: a selective radical pathway. J. Am. Chem. Soc.141, 11212–11218 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, W. et al. Methylcyclopentenyl cations linking initial stage and highly efficient stage in methanol-to-hydrocarbon process. ACS Catal.10, 4510–4516 (2020). [Google Scholar]
- 15.Chen, W., Han, J., Wei, Y. & Zheng, A. Frustrated lewis pair in zeolite cages for alkane activations. Angew. Chem., Int. Ed. Engl.61, e202116269 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Chen, W., Yi, X., Liu, Z., Tang, X. & Zheng, A. Carbocation chemistry confined in zeolites: spectroscopic and theoretical characterizations. Chem. Soc. Rev., 10.1039/d1cs00966d (2022). [DOI] [PubMed]
- 17.Wang, S. et al. Catalytic roles of the acid sites in different pore channels of H-ZSM-5 zeolite for methanol-to-olefins conversion. Chin. J. Catal.42, 1126–1136 (2021). [Google Scholar]
- 18.Van der Mynsbrugge, J., De Ridder, J., Hemelsoet, K., Waroquier, M. & Van Speybroeck, V. Enthalpy and entropy barriers explain the effects of topology on the kinetics of zeolite-catalyzed reactions. Chem. Eur. J.19, 11568–11576 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, J. et al. Hydrogen transfer versus olefins methylation: on the formation trend of propene in the methanol-to-hydrocarbons reaction over Beta zeolites. J. Catal.368, 248–260 (2018). [Google Scholar]
- 20.Wen, W. et al. Formation and Fate of Formaldehyde in Methanol-to-Hydrocarbon Reaction: In Situ Synchrotron Radiation Photoionization Mass Spectrometry Study. Angew. Chem., Int. Ed. Engl.59, 4873–4878 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Muller, S. et al. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. J. Am. Chem. Soc.138, 15994–16003 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Kilburn, L., DeLuca, M., Hoffman, A. J., Patel, S. & Hibbitts, D. Comparing alkene-mediated and formaldehyde-mediated diene formation routes in methanol-to-olefins catalysis in MFI and CHA. J. Catal.400, 124–139 (2021). [Google Scholar]
- 23.Fang, X. et al. Highly enhanced aromatics selectivity by coupling of chloromethane and carbon monoxide over H-ZSM-5. Angew. Chem., Int. Ed. Engl.61, e202114953 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Chen, Z. et al. Coupling of methanol and carbon monoxide over H-ZSM-5 to form aromatics. Angew. Chem., Int. Ed. Engl.57, 12549–12553 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Jackson, J. E. & Bertsch, F. M. Conversion of methanol to gasoline: new mechanism for formation of the first carbon-carbon bond. J. Am. Chem. Soc.112, 9085–9092 (1990). [Google Scholar]
- 26.Chen, W. et al. Molecular understanding of the catalytic consequence of ketene intermediates under confinement. J. Am. Chem. Soc.143, 15440–15452 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Z. et al. CO cofeeding affects product distribution in CH3Cl coupling over ZSM‐5 zeolite: pressure twists the plot. Angew. Chem. Int. Ed. 63, 10.1002/anie.202401060 (2024). [DOI] [PubMed]
- 28.Cesarini, A. et al. Elucidation of radical- and oxygenate-driven paths in zeolite-catalysed conversion of methanol and methyl chloride to hydrocarbons. Nat. Catal., 10.1038/s41929-022-00808-0 (2022). [DOI] [PMC free article] [PubMed]
- 29.Jiao, F. et al. Selective conversion of syngas to light olefins. Science351, 1065–1068 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y. et al. Formation mechanism of the first carbon–carbon bond and the first olefin in the methanol conversion into hydrocarbons. Angew. Chem.128, 5817–5820 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury, A. D. et al. Bridging the gap between the direct and hydrocarbon pool mechanisms of the methanol‐to‐hydrocarbons process. Angew. Chem.130, 8227–8231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailleul, S. et al. Ab initio enhanced sampling kinetic study on MTO ethene methylation reaction. J. Catal.388, 38–51 (2020). [Google Scholar]
- 33.Chen, Q., Liu, J. & Yang, B. Microenvironment of the HMOR catalyst leads to high ethylene selectivity from ketene conversion: Insights from ab initio molecular dynamics simulations. Chem. Eng. J.481, 148412 (2024). [Google Scholar]
- 34.Chowdhury, A. D. & Gascon, J. The curious case of ketene in zeolite chemistry and catalysis. Angew. Chem., Int. Ed. Engl.57, 14982–14985 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury, A. D. et al. Bridging the gap between the direct and hydrocarbon pool mechanisms of the methanol-to-hydrocarbons process. Angew. Chem., Int. Ed. Engl.57, 8095–8099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, L. et al. Probing into the building and evolution of primary hydrocarbon pool species in the process of methanol to olefins over H-ZSM-5 zeolite. Mol. Catal. 516, 10.1016/j.mcat.2021.111968 (2021).
- 37.Hu, M. et al. Establishing a link between the dual cycles in methanol-to-olefins conversion on H-ZSM-5: aromatization of cycloalkenes. ACS Catal.10, 4299–4305 (2020). [Google Scholar]
- 38.Wang, S. et al. Origin and evolution of the initial hydrocarbon pool intermediates in the transition period for the conversion of methanol to olefins over H-ZSM-5 zeolite. J. Catal.369, 382–395 (2019). [Google Scholar]
- 39.Plessow, P. N. & Studt, F. Unraveling the mechanism of the initiation reaction of the methanol to olefins process using ab initio and DFT calculations. ACS Catal.7, 7987–7994 (2017). [Google Scholar]
- 40.Liu, Y. et al. Formation mechanism of the first carbon-carbon bond and the first olefin in the methanol conversion into hydrocarbons. Angew. Chem., Int. Ed. Engl.55, 5723–5726 (2016). [DOI] [PubMed] [Google Scholar]
- 41.DeLuca, M., Janes, C. & Hibbitts, D. Contrasting arene, alkene, diene, and formaldehyde hydrogenation in H-ZSM-5, H-SSZ-13, and H-SAPO-34 frameworks during MTO. ACS Catal.10, 4593–4607 (2020). [Google Scholar]
- 42.Dong, Z. et al. Understanding the structure–activity relationships in catalytic conversion of polyolefin plastics by zeolite-based catalysts: a critical review. ACS Catal.12, 14882–14901 (2022). [Google Scholar]
- 43.Fan, S. et al. Formation and evolution of methylcyclohexene in the initial period of methanol to olefins over H-ZSM-5. ACS Catal., 12477–12487, 10.1021/acscatal.2c03410 (2022).
- 44.Wei, C., Yu, Q., Li, J. & Liu, Z. Coupling Conversion of n-Hexane and CO over an HZSM-5 Zeolite: Tuning the H/C Balance and Achieving High Aromatic Selectivity. ACS Catal.10, 4171–4180 (2020). [Google Scholar]
- 45.Yang, L. et al. Role of acetaldehyde in the roadmap from initial carbon–carbon bonds to hydrocarbons during methanol conversion. ACS Catal.9, 6491–6501 (2019). [Google Scholar]
- 46.Enss, A. E., Huber, P., Plessow, P. N. & Studt, F. Methanol-mediated hydrogen transfer reactions at surface lewis acid sites of H-SSZ-13. J. Phys. Chem. C.128, 15367–15379 (2024). [Google Scholar]
- 47.Liu, Z., Dong, X., Liu, X. & Han, Y. Oxygen-containing coke species in zeolite-catalyzed conversion of methanol to hydrocarbons. Catal. Sci. Technol.6, 8157–8165 (2016). [Google Scholar]
- 48.Chen, Z. et al. The carboxylates formed on oxides promoting the aromatization in syngas conversion over composite catalysts. Chin. J. Catal.42, 835–843 (2021). [Google Scholar]
- 49.Yang, J., Pan, X., Jiao, F., Li, J. & Bao, X. Direct conversion of syngas to aromatics. Chem. Commun.53, 11146–11149 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Kuhne, T. D. et al. CP2K: An electronic structure and molecular dynamics software package - quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys.152, 194103 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
- 52.Yang, K., Zheng, J., Zhao, Y. & Truhlar, D. G. Tests of the RPBE, revPBE, tau-HCTHhyb, omegaB97X-D, and MOHLYP density functional approximations and 29 others against representative databases for diverse bond energies and barrier heights in catalysis. J. Chem. Phys.132, 164117 (2010). [DOI] [PubMed] [Google Scholar]
- 53.VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys.127, 114105 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem.32, 1456–1465 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Schroder, H., Creon, A. & Schwabe, T. Reformulation of the D3(Becke-Johnson) dispersion correction without resorting to higher than C(6) dispersion coefficients. J. Chem. Theory Comput.11, 3163–3170 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Rey, J., Raybaud, P., Chizallet, C. & Bučko, T. Competition of secondary versus tertiary carbenium routes for the type B Isomerization of alkenes over acid zeolites quantified by ab initio molecular dynamics simulations. ACS Catal.9, 9813–9828 (2019). [Google Scholar]
- 57.Leung, K., Nielsen, I. M. B. & Criscenti, L. J. Elucidating the bimodal acid−base behavior of the water−silica interface from first principles. J. Am. Chem. Soc.131, 18358–18365 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Herron, J. A., Morikawa, Y. & Mavrikakis, M. Ab initio molecular dynamics of solvation effects on reactivity at electrified interfaces. Proc. Natl. Acad. Sci. USA. 113, 10.1073/pnas.1604590113 (2016). [DOI] [PMC free article] [PubMed]
- 59.Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys.52, 255–268 (1984). [Google Scholar]
- 60.Barducci, A., Bonomi, M. & Parrinello, M. Metadynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci.1, 826–843 (2011). [Google Scholar]
- 61.Laio, A. & Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA.99, 12562–12566 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen, Q., Liu, J. & Yang, B. Identifying the key steps determining the selectivity of toluene methylation with methanol over HZSM-5. Nat. Commun.12, 3725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van der Mynsbrugge, J., Moors, S. L. C., De Wispelaere, K. & Van Speybroeck, V. Insight into the formation and reactivity of framework-bound methoxide species in H-ZSM-5 from static and dynamic molecular simulations. ChemCatChem6, 1906–1918 (2014). [Google Scholar]
- 64.Van Speybroeck, V. et al. First principle chemical kinetics in zeolites: the methanol-to-olefin process as a case study. Chem. Soc. Rev.43, 7326–7357 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Moors, S. L. C., De Wispelaere, K., Van der Mynsbrugge, J., Waroquier, M. & Van Speybroeck, V. Molecular dynamics kinetic study on the zeolite-catalyzed benzene methylation in ZSM-5. ACS Catal.3, 2556–2567 (2013). [Google Scholar]
- 66.De Wispelaere, K., Bailleul, S. & Van Speybroeck, V. Towards molecular control of elementary reactions in zeolite catalysis by advanced molecular simulations mimicking operating conditions. Catal. Sci. Technol.6, 2686–2705 (2016). [Google Scholar]
- 67.Fu, H. et al. Finding an optimal pathway on a multidimensional free-energy landscape. J. Chem. Inf. Model.60, 5366–5374 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Baerlocher, C. & McCusker, L. B. Database of Zeolite Structures, http://www.iza-structure.org/databases/ (2023).
- 69.Wang, S. et al. Relation of catalytic performance to the aluminum siting of acidic zeolites in the conversion of methanol to olefins, viewed via a comparison between ZSM-5 and ZSM−11. ACS Catal.8, 5485–5505 (2018). [Google Scholar]
- 70.Hansen, N., Kerber, T., Sauer, J., Bell, A. T. & Keil, F. J. Quantum chemical modeling of benzene ethylation over H-ZSM-5 approaching chemical accuracy: a hybrid MP2:DFT Study. J. Am. Chem. Soc.132, 11525–11538 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are provided in the Supplementary Information file.