Abstract
Hypoxic vasodilation is a fundamental, highly conserved physiological response that requires oxygen and/or pH sensing coupled to vasodilation. While this process was first characterized more than 80 years ago, the precise identity and mechanism of the oxygen sensor and mediators of vasodilation remain uncertain. In support of a possible role for hemoglobin (Hb) as a sensor and effector of hypoxic vasodilation, here we show biochemical evidence that Hb exhibits enzymatic behavior as a nitrite reductase, with maximal NO generation rates occurring near the oxy-to-deoxy (R-to-T) allosteric structural transition of the protein. The observed rate of nitrite reduction by Hb deviates from second-order kinetics, and sigmoidal reaction progress is determined by a balance between 2 opposing chemistries of the heme in the R (oxygenated conformation) and T (deoxygenated conformation) allosteric quaternary structures of the Hb tetramer — the greater reductive potential of deoxyheme in the R state tetramer and the number of unligated deoxyheme sites necessary for nitrite binding, which are more plentiful in the T state tetramer. These opposing chemistries result in a maximal nitrite reduction rate when Hb is 40–60% saturated with oxygen (near the Hb P50), an apparent ideal set point for hypoxia-responsive NO generation. These data suggest that the oxygen sensor for hypoxic vasodilation is determined by Hb oxygen saturation and quaternary structure and that the nitrite reductase activity of Hb generates NO gas under allosteric and pH control.
Introduction
Hypoxic vasodilation is a fundamental physiological response that ensures adequate oxygen delivery and blood buffering capacity (including CO2 elimination) to tissues under metabolic stress. This highly conserved physiological response requires oxygen and pH sensing coupled with vasodilation. While this process was first characterized more than 80 years ago (1, 2), the precise identity and mechanism of the oxygen sensor and mediators of vasodilation remain unknown (3, 4). From an oxygen-sensing standpoint, a situation in which the metabolic demand of a tissue exceeds oxygen delivery causes a divergence from the normal relationship between tissue oxygen consumption and vascular oxygen delivery. This creates an “error signal” that is characterized by an increasingly negative slope when venous PaO2 is plotted against oxygen consumption (4). While conventional wisdom suggests that this error signal is detected as a change in tissue or blood partial pressure of oxygen (PO2) (i.e., the oxygen sensor detects PO2), recent studies have suggested that Hb oxygen saturation and Hb’s highly evolved allosteric conformational changes may subserve this oxygen-sensor function (5–13). Such allosteric changes have been associated with the release of red blood cell ATP (5–8), the formation of S-nitrosothiols from S-nitrosated Hb (9, 10), and, recently, the reduction of blood nitrite to NO by Hb (11–14).
Early studies by Furchgott, Ignarro, and Murad demonstrated the ability of nitrite to activate soluble guanylate cyclase (sGC) and vasodilate aortic rings (15–17). However, the concentrations required for this effect in vitro (in the hundred-μM to mM range) largely precluded consideration of a role for nitrite in vivo in humans where circulating concentrations are typically less than 1 μM. We have reported the existence of arterial-to-venous gradients of plasma nitrite that increase with regional metabolic stress (18) and recently described a vasodilatory activity of nitrite at near physiological concentrations in vivo (11). This vasodilatory activity was associated with the conversion of nitrite to NO by deoxyHb, suggesting that Hb possesses a physiological nitrite reductase activity that might participate in hypoxic vasodilation. Indeed, Brooks in 1937 and Doyle and colleagues in 1981 characterized the reduction of nitrite to NO by deoxyHb (19, 20).
Reaction
 |
1 |
We were struck by the potential physiological relevance of this reaction. The requirements for deoxyHb and protons in the reaction suggested a potential mechanism for oxygen and pH sensing. Furthermore, the 1-electron reduction of nitrite by ferrous deoxyheme suggests that redox properties of the heme may modulate this process. In support of these ideas, data from kinetic in vitro aortic ring bioassay experiments have demonstrated an apparent vasodilation threshold whereby nitrite-Hb–dependent vasodilation is initiated at a PO2 approximating the Hb P50 (11). The P50 represents the PO2 at which Hb is 50% saturated with oxygen. Importantly, as demonstrated in this study, the rate of nitrite reduction to NO gas by Hb is maximal when Hb is 50% saturated with oxygen. A maximal nitrite reductase activity occurring at the Hb P50 would be ideal for hypoxic vasodilation, as the Hb P50 is thermally, chemically, and allosterically modulated by surrounding tissue metabolism (21), and classical physiological studies of hypoxic vasodilation reveal an apparent hypoxic vasodilation threshold at 40–60% Hb-oxygen saturation (4, 22).
Results
The nitrite reductase reaction revisited.
The reaction of nitrite and deoxyHb has previously been described as a first-order reaction with respect to deoxyHb under conditions of excess nitrite, generating unbalanced product yields of 72% metHb and 28% iron-nitrosyl-Hb (19). However, based on Reaction 1, equivalent (50:50%) product yields would be expected, with 1 mole of nitrite generating 1 mole of metheme and 1 mole of iron-nitrosyl-heme. We have examined this reaction under strict anaerobic conditions in the presence of excess nitrite (50 μM heme and 10 mM nitrite) and observed an unexpected divergence from first-order kinetics and equimolar product yields of metheme and iron-nitrosyl-heme (Figure 1, A–C). However, with the introduction of trace oxygen (18% oxyheme present at the beginning of the reaction), the rate of the reaction increases and more closely (although never exactly) approximates the first-order behavior observed in earlier studies (Figure 1, A and B). The nitrite reaction with partially oxygenated Hb also produces metheme and iron-nitrosyl-heme product yields consistent with levels observed in earlier studies (Figure 1C) (23). An oxygen leak would be expected to increase the yield of metheme due to the simultaneous reaction of nitrite with oxyheme as well as the reaction of NO (formed from the reaction of nitrite with deoxyheme) with oxyheme.
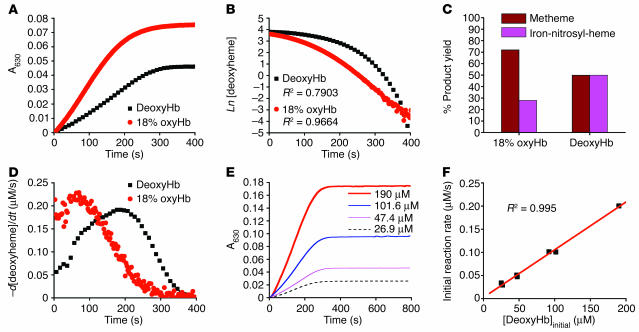
Figure 1.
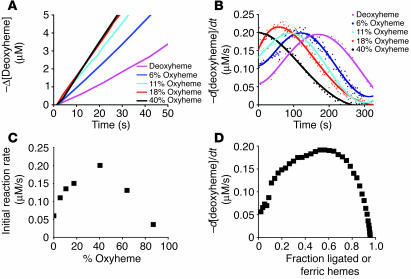
Reaction of nitrite with deoxyHb reveals deviation from first-order kinetics and equimolar product yields of metHb and iron-nitrosyl-Hb. (A) Progress of the nitrite-Hb reaction under conditions of excess nitrite (10 mM nitrite with 50 μM heme) monitored spectrophotometrically by formation of ferric hemes at 630 nm (baseline at 700 nm) under anaerobic conditions or during partial oxygenation (18% oxyHb). 50 μM metHb gives A630 of 0.12 with 10 mM nitrite present. (B) The fit of the natural log of deoxyheme concentration versus time for conditions shown in A, where Ln[deoxyheme] is the natural log of the deoxyheme concentration at each time point. The deoxyheme concentration at each time point was determined by spectral deconvolution. (C) Effects of oxygen leak on product yields in the nitrite-Hb reaction (calculated by spectral deconvolution for reactions in A). (D) Instantaneous rate of deoxyheme consumption over time during the course of a single reaction under the conditions in A. The instantaneous rate of deoxyheme consumption was found by negative change of deoxyheme concentration between 2 adjacent time points (–d[deoxyheme]) over the time interval (dt) where d is change and t is time. (E) Progress of the reaction of nitrite (10 mM) with varying initial concentrations of deoxyheme monitored by the formation of metheme at 630 nm. (F) Effect of varying the initial deoxyheme concentration (concentration of deoxyheme at the beginning of the reaction; [DeoxyHb]initial) on the initial rate of the reaction calculated from the data in E. The initial reaction rate is the rate of ferric heme formation at the beginning of the reaction calculated as the average rate over the first 200 seconds using extinction coefficient of 3.4 at 630 nm (the rate of deoxyheme consumption is approximately twice as fast).
However, the deviation from first-order kinetics in the absence of oxygen and the effect of oxygen on increasing the reaction rate were unexpected (Figure 1, B and D). On initial inspection, under conditions in which nitrite concentration exceeded deoxyheme, the reaction of nitrite with deoxyheme (Figure 1A) appeared to display zero-order kinetics with respect to deoxyheme, as evidenced by a relatively constant rate throughout the process of the reaction even though the concentration of deoxyheme was continually decreasing (Reaction 1 and Figure 1, A and E). However, when we increased the initial concentration of deoxyHb (in the range of 26.9 μM to 190 μM) we observed a linear increase in the initial reaction rate (Figure 1, E and F), a property consistent with first-order kinetics with respect to deoxyheme. A more careful examination of Figure 1, A and D, suggests a sigmoidal reaction profile characterized by an increase and then a decrease in the rate, with the maximal rate occurring when approximately 50% of the deoxyheme is converted to metheme and iron-nitrosyl-heme. Thus, despite the decreasing concentration of deoxyheme available to react with nitrite during the course of the reaction, the reaction rate is able to increase until it reaches its maximum.
Increased rate of nitrite reduction by R state Hb.
We hypothesized that the sigmoidal reaction profile observed is the sum of 2 opposing reactions of nitrite with deoxyheme in the 2 allosteric conformations of Hb. As the reaction progresses, the reaction rate of nitrite with deoxyheme in T state tetramer decreases as deoxyheme is consumed. This is balanced by the accelerating reaction of nitrite with R state deoxyheme, as the concentration of this allosteric conformation increases with the formation of metheme and iron-nitrosyl-heme, which both stabilize R state tetramer (Figure 2A). This causes a T-to-R state allosteric transition to occur as metheme and iron-nitrosyl-heme levels increase in the Hb tetramer. We measured the bimolecular rate constant of the reaction of nitrite with deoxyHb and myoglobin (Mb) over time and found that this parameter remained constant for monomeric Mb (Figure 2B). In contrast, the bimolecular rate constant increased exponentially (R2 = 0.998 for exponential fit) over the course of the reaction of tetrameric deoxyHb with nitrite, supporting the model in which the nitrite reductase activity of tetrameric deoxyHb increases during the reaction time course (Figure 2B). We confirmed the presence of a T-to-R allosteric transition during the course of the reaction by monitoring the stabilization of the bond between the proximal histidine and the NO-liganded heme in the α chain of Hb by electron paramagnetic resonance (EPR) spectroscopy. As shown in Figure 2, C and D (experiment performed using 100 μM deoxyheme and 2.5 mM nitrite), as the reaction proceeded, the α iron-nitrosyl-heme transitioned from 5 (T state) to 6 (R state) coordinated structure. This occurred because T state α iron-nitrosyl-heme exists in equilibrium between a 5-coordinate (histidine-to-heme bond disrupted) and 6-coordinate (histidine bond intact) species, while R state α iron-nitrosyl retains 100% 6-coordinate heme geometry (24). The percentage yield of each iron-nitrosyl-heme species during the reaction time course was similar to that observed by Hille et al. when NO buffer was titrated into deoxyHb solution (25). Also, the iron-nitrosyl-heme spectral change observed during the reaction process, as shown in Figure 2C, was similar to the spectral change observed during deoxygenation and reoxygenation of substoichiometric iron-nitrosyl-Hb (26). This confirms that as the deoxyheme is converted to metheme and iron-nitrosyl-heme during the reaction, the T-to-R allosteric transition does indeed occur.
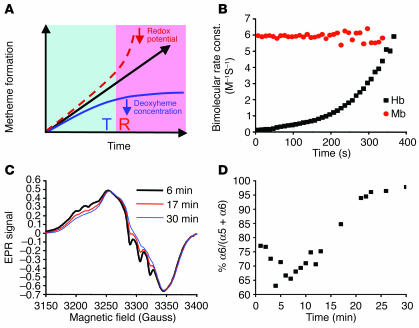
Figure 2.
Sigmoidal reaction behavior of the nitrite-deoxyheme reaction occurs due to T-to-R allosteric quaternary transition of Hb. (A) Model of the nitrite-deoxyHb reaction representing a balance between 2 opposing processes: (a) a decelerating reaction with T state deoxyheme due to depletion of deoxyheme available for reaction with nitrite because of conversion to metheme and iron-nitrosyl-heme (solid blue line); and (b) an accelerating reaction of nitrite with R state deoxyheme (dashed line). Solid black line with arrow represents the reaction process observed experimentally and is the balance of these 2 processes. (B) Apparent bimolecular rate constant over the time course of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations). Bimolecular rate constant (const.) was obtained by dividing the instantaneous reaction rate of deoxyheme consumption by the concentration of deoxyheme and nitrite. (C) Initial (6 minutes), intermediate (17 minutes), and final (30 minutes) EPR spectra of iron-nitrosyl-heme monitored over the course of the nitrite-deoxyHb reaction (100 μM heme, 2.5 mM nitrite) showing a transition from 5-coordinate (T state) α iron-nitrosyl-heme (with characteristic hyperfine splitting) to 6-coordinate (R state) heme geometry. The smaller EPR signals at earlier time points were normalized to that of the final time point in order to compare the spectral shape of the iron-nitrosyl-Hb signal. (D) T-to-R allosteric structural transition during the course of the nitrite-deoxyHb reaction, monitored by the percentage formation of 6-coordinate α iron-nitrosyl-heme relative to total α iron-nitrosyl-heme.
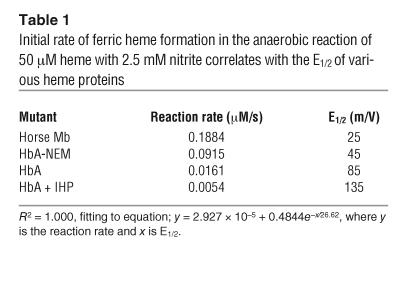
It is likely that the accelerating rate of reaction of nitrite with R state Hb occurs due to the lower reduction potential (hence, a better electron donor) of the deoxyheme within the R state versus T state tetramers (14, 27). The initial rates of reaction of nitrite with Mb (R state monomer), N-ethylmaleimide–modified (NEM-modified) deoxyHb (R state stabilizing effect), native Hb A (HbA), and inositol hexaphosphate–treated (IHP-treated) Hb (T state stabilizing) were compared to their published reduction potentials (E1/2s) as averaged for the subunits to obtain overall values for the Hb tetramer (50 μM heme, 2.5 mM nitrite) (Table 1) (27, 28). Similar to the initial rates plotted in Figure 1F, the initial rate of the reaction indicated in Table 1 was measured by the average rate of metHb formation using the initial part of the reaction trace at 630 nm. A higher rate of nitrite reduction correlated with a higher oxygen affinity or R state character and with lower E1/2s for all of these conditions (R2 = 1.000 for exponential fit). These data are also consistent with the observed correlation between higher heme reduction potentials and the rates of alkaline reductive nitrosylation of metHb by NO, which forms ferrous Hb and nitrite; this reaction can be considered the reverse of the nitrite reductase reaction and is accelerated by higher heme E1/2 and alkaline pH (29). These data confirm that deoxyheme in the R state tetrameric Hb is a more facile nitrite reductase due to lowered heme E1/2.
Table 1.
Initial rate of ferric heme formation in the anaerobic reaction of 50 μM heme with 2.5 mM nitrite correlates with the E1/2 of various heme proteins
It is clear from these data that the deviation from first-order kinetics in the reaction of nitrite with deoxyHb occurs because of the T-to-R allosteric transition. This was also confirmed by the first-order kinetics of molecules that are incapable of allosteric transition, such as Mb and β chains of Hb (R state tetramer only) and Hb with IHP and low pH (locked in T state). These data are shown for Mb in Figure 3, A and B, and for IHP at low pH and the β chains of HbA in Figure 3, E and F.
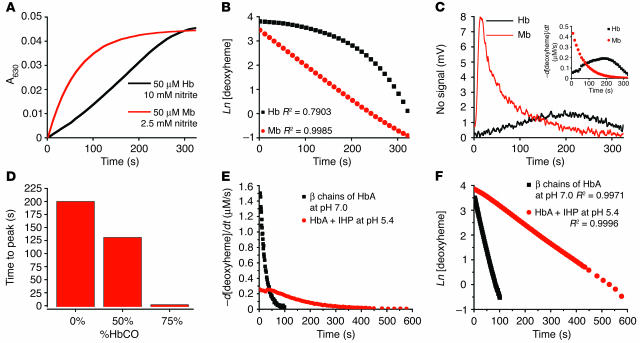
Figure 3.
Rate of nitrite reductase reaction and NO gas formation is under allosteric control. (A) Progress of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations) monitored spectrophotometrically by metHb formation at 630 nm. (B) First order fits for Mb and non–first order behavior of tetrameric Hb (fits of natural log of deoxyheme concentration for the same reactions shown in A). (C) Simultaneous measurement of NO gas by chemiluminescence during the course of the reaction shown in A. Inset shows the instantaneous rate of deoxyheme consumption over the course of the reaction, obtained from spectral deconvolution. (D) The time to peak NO production measured by chemiluminescence for the reaction of nitrite (10 mM) with Hb (50 μM heme) with varying saturation (0–75%) of carbon monoxide. %HbCO, percentage of Hb that is saturated with carbon monoxide. (E) Reaction progress for β chains of HbA (locked in R state tetramer; 35 μM heme reacted with 0.5 mM nitrite at pH 7.0) and IHP-treated Hb (locked into T state tetramer; 50 μM heme reacted with 2.5 mM nitrite at pH 6.4) was monitored by the rate of deoxyheme consumption. (F) First-order fits for β chains of HbA at pH 7.0 and for IHP-treated Hb at pH 6.4 for conditions in E showing that the deviation from first order requires an allosteric structural transition of the Hb tetramer (fits of natural log of deoxyheme concentration for the same reactions shown in E).
For tetrameric Hb reacting anaerobically with nitrite, the reaction is essentially an allosteric autocatalytic reaction with a single nitrite reacting with one T state heme to generate a metheme and an iron-nitrosyl-heme, which by virtue of their R state stabilizing effects, allosterically lower the redox potential of 2–6 other hemes (dependent on inter- or intratetrameric iron-nitrosyl formation). This produces autocatalysis as R state hemes generate more R state hemes, exponentially decreasing E1/2 and increasing bimolecular rate constants during the course of the reaction (Figure 2B). Two recent papers have explored the reaction of nitrite with deoxyHb, coming to different conclusions. A study by Luchsinger and colleagues reported that this reaction yielded product ratios of metHb to iron-nitrosyl-Hb of 1:1 to 1:6, consistent with earlier studies by Doyle, and that the reaction appeared to obey first-order kinetics (19, 30). However, in the Luchsinger study, the initial deoxyHb preparation contained approximately 10% metHb; this contamination produces R state catalysis of this reaction. A second study by Huang et al. demonstrated the effects of metHb and R state stabilization on increasing the reaction rate and mathematically modeled the nitrite-deoxyHb reaction, producing results that are consistent with our proposed allosteric autocatalytic reaction (14).
Maximal NO generation near the Hb P50 provides Hb with oxygen-sensor and NO-donor properties.
The above considerations suggest that the rate of the nitrite-deoxyHb reaction is maximal at 40–60% oxygen saturation because of the balance between the deoxyheme concentration, which is required for nitrite binding, and the greater reductive ability of the R state deoxyheme, which is required for the reduction of nitrite. To confirm this, we next measured NO gas formation during the nitrite-Hb and nitrite-Mb reaction and tested to see whether NO generation is under allosteric and proton-linked control. In these experiments, deoxygenated Mb, Hb, and partially carbon monoxide– or oxygen-bound Hb preparations were reacted with excess nitrite anaerobically, while constant stirring and purging with helium occurred (except for partial oxyHb reactions). The purged gas traveled in-line into a chemiluminescent NO gas analyzer for detection of NO. The reaction was monitored simultaneously by visible absorbance spectrophotometry and the rate of reaction measured as the rate of deoxyheme consumption.
Mb (50 μM) is locked in R state conformation and has a low heme reduction potential. As expected, the reaction with excess nitrite (2.5 mM) exhibited a rapid rate with first-order exponential decay as the deoxyheme sites were consumed in the reaction (Figure 3, A and B). Simultaneously measured NO gas generation followed similar first-order kinetics with a rapid initial rate of NO formation that decreased exponentially (consistent with a first-order process; Figure 3C). In contrast, tetrameric Hb exhibited a more sigmoidal reaction profile, thus deviating from first-order behavior, and demonstrated a maximal rate of NO generation in the middle of the reaction when the balance between the number of deoxyhemes and their reductive potential was optimal (Figure 3, A–C). Since the ligation of CO to heme produces the R state conformation of Hb, we equilibrated deoxyHb with varying concentrations of CO and reacted this partial carboxyHb mixture (0, 50, and 75%) with nitrite to evaluate the effect of R state character on the rate of NO generation. As shown in Figure 3D, the time to peak NO production decreased as carboxyHb saturation increased from 0 to 75%, corresponding with increasing R state character at the beginning of the reaction. β chains of HbA were locked in R state tetrameric conformation, and IHP-treated HbA at low pH was locked in T state tetrameric conformation. The rate of deoxyheme consumption for both of these Hb species, similar to that for Mb (as shown in Figure 3C inset), was fast initially and then slowed down exponentially as their reaction with nitrite progressed (Figure 3E). Together with the Mb’s linear first-order fit, shown in Figure 3B, the first-order fits for β chains and IHP-treated Hb demonstrate that in the absence of an allosteric structural transition of the Hb tetramer, the reaction follows first-order kinetics (Figure 3F). We also confirmed the more rapid rate of reaction for the R state β chains of Hb compared with the slower rate for the T state HbA in the presence of IHP at low pH.
Maximal nitrite reductase rate is observed at 40–60% oxyHb saturation.
We next added nitrite to Hb with a range of initial oxyHb saturations and monitored the initial rate of the nitrite reductase reaction by deconvoluting the deoxyHb spectra and observing the rate of disappearance of deoxyheme. This allowed for the specific measurement of the nitrite reductase reaction independent of additional reactions between nitrite and oxyheme. These reactions were performed in anaerobic cuvettes with additions of oxygen to achieve the measured oxygen saturations shown in Figure 4. Consistent with allosteric modulation of the nitrite reductase reaction, the initial rate of the reaction increased as the initial oxyHb concentration increased and reached a maximal rate at 40–60% Hb-oxygen saturation (Figure 4, A–C). We reexamined the anaerobic reaction of nitrite with deoxyHb by plotting the instantaneous reaction rate at each time point during the course of the reaction versus the fraction of total hemes ligated with NO and oxidized to metHb. This revealed a similar effect: a maximal nitrite reductase rate at approximately 40–60% Hb ligation (in this case metheme and iron-nitrosyl acting as R state modifiers; Figure 4D).
Figure 4.
Maximal rates for nitrite reduction to NO occurs around the Hb P50. (A) The negative of the change in deoxyheme concentration (obtained by spectral deconvolution) over time during the reaction of nitrite (10 mM) with partially oxygenated (0–40%) Hb (50 μM total heme). (B) Instantaneous rate of deoxyheme consumption over the course of the experiment described in A. (C) Initial rate (intercept of the polynomial fitting in B at 0 seconds) of reaction for the conditions described above plotted as a function of initial oxygen saturation. (D) The instantaneous rate of deoxyheme consumption versus fraction ligated or oxidized ferric hemes (relative to total heme: deoxy-, met-, and iron-nitrosyl-Hb) during the course of the anaerobic reaction of nitrite (10 mM) with deoxyheme (50 μM).
pH sensor chemistry is mediated by nitrite protonation and redox Bohr effect.
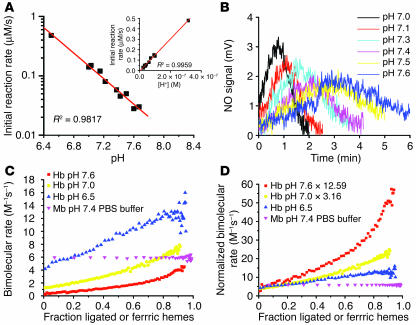
Because the nitrite reduction reaction described in Reaction 1 requires a proton, increasing concentration of protons would be expected to accelerate this reaction by the inverse log of –ΔpH (i.e., as pH decreases from 7.6 to 6.5 the rate should increase by 10–(ΔpH) = 10–(6.5 – 7.6) = 101.1 or by 12.59 fold). However, if our allosteric model of nitrite reduction is correct, an additional opposing “redox (oxidation) Bohr effect” of decreasing pH on the stabilization of the T state tetramer would be expected to increase the heme redox potential and slow down the reaction. Analogous to the oxygen Bohr effect of Hb (31, 32), the relationship between redox potential of ferrihemes and pH is linear within the physiologic pH range, such that a 1-unit decrease in pH is accompanied by a 60 mV increase in E1/2 (33–36). To characterize these competing chemistries, establish whether the nitrite reductase activity of Hb has pH sensor properties, and provide additional confirmation of an allosteric model, we evaluated the effect of pH on this reaction using 0.01 M phosphate buffer adjusted to the corresponding pH. Consistent with the inverse log increase in rate (10–ΔpH) due to more nitrite protonation, decreasing pH within the physiological range (pH 6.4–7.8) dramatically increased the initial rate of the reaction (measured by spectral deconvolution, R2 = 0.98 for linear fit of log10 [initial rate] vs. pH) and the rate of NO gas generation from nitrite (measured by chemiluminescence) (Figure 5, A and B).
Figure 5.
Effect of pH on the nitrite reductase reaction. (A) The initial rate (found by the polynomial fitting of the rate of deoxyheme consumption over fraction ligated) of the reaction of nitrite (2.5 mM) with deoxyHb (45 μM heme) at different pH values in 0.01 M phosphate buffer. Inset shows the initial rate plotted against concentration of proton. (B) NO gas measured by chemiluminescence from the reactions shown in A. (C) The bimolecular rate of the reaction of nitrite with deoxyHb in phosphate buffer at pH 7.6, 7.0, and 6.5 and deoxymyoglobin in PBS buffer at pH 7.4 over heme ligand and oxidation states. (D) Correction factors 12.59 and 3.16 (given by 10pH – 6.5) were multiplied by the bimolecular rates at pH 7.6 and 7.0, respectively (shown in C) to eliminate the contribution of changing proton concentrations to the bimolecular rate.
It is important to recognize that the initial reaction rate could not be affected by the redox Bohr effect because all of the Hb was already fully deoxygenated and in T state at the beginning of the reaction. This is clearly evidenced by the exact inverse log increase in initial reaction rate as pH decreased (Figure 5A) or, equivalently, the linear increase in the initial reaction rate as proton concentration increased (Figure 5A, inset). The initial reaction rate increased as the proton concentration increased, and the linear fit extended through the zero point, demonstrating that protonation is required for the nitrite and deoxyHb reaction (Figure 5A, inset). However, we hypothesized that as the reaction proceeded, the redox Bohr effect would slow the transition from T-to-R by stabilizing the T state. We therefore compared the “instantaneous” bimolecular rate of the reaction at pH values of 7.6, 7.0, and 6.5 during the course of the reaction, over a range of heme “saturations” (i.e., data shown as the fraction of total NO-ligated or ferric hemes during the process of the anaerobic reaction of nitrite and Hb). In Figure 5C, we indicate the expected large effect of decreasing pH on increasing the initial bimolecular rate (calculated as instantaneous reaction rate divided by the concentration of nitrite and deoxyheme), but the increase in the bimolecular rate as the Hb becomes fully ligated and undergoes T-to-R transition was reduced. This is more clearly shown by normalizing the bimolecular rates during the course of the reaction by the inverse log of –ΔpH to correct for the direct proton concentration effect. In Figure 5D, we clearly show that the initial bimolecular rate is unaffected by redox Bohr effect, as all the Hb is in the T state, but as the reaction progresses, the rise in the bimolecular rate is limited at low pH due to the effect of redox Bohr on the stabilization of T state tetramer. In a physiological system, the effect of lowering pH on increasing the heme redox potential would be more than offset by the inverse log increase in the reaction rate and pH-dependent exposure of more deoxyhemes for nitrite reduction.
Kinetic considerations.
The chemical behavior of Hb as a nitrite reductase presents an elegant mechanism for oxygen sensing, as the maximal nitrite reductase activity is allosterically linked to the Hb P50. In addition, this mechanism appears to address kinetic concerns over the requirement for a rapid generation of a vasodilating effector. While the bimolecular rate constant of nitrite reduction by T state deoxyHb is 0.12 M–1s–1 at 25°C and pH 7.4, this bimolecular rate constant increases exponentially during T-to-R allosteric transition to approximately 50-fold at the end of the reaction (Figure 2B). Thus the rate of the R4 state Hb tetramer approaches that of Mb (6 M–1s–1; Figure 2B). The average bimolecular rate constant during the reaction process was 0.35 M–1s–1, on the same order as our previously measured value of 0.47 M–1s–1 (11) and that found by Doyle et al. (1 M–1s–1) (19). In addition, the requirement of a proton for the reaction suggests that the rate will increase by the inverse log of -ΔpH. For example, in a vascular bed under physiological metabolic stress with Hb oxygen saturation of 60% at pH 7.0 and 37°C, the bimolecular rate of nitrite reduction to NO would be 4.4 M_1s_1. We and others have recently reported that red blood cells have 300 nM intracellular nitrite in steady state and that these levels are controlled across a narrow physiological range (37). Indeed, blood levels of nitrite appear to be conserved across species with uptake and utilization by the erythrocyte under oxygen- and pH-dependent control (18, 38–40). As Hb deoxygenates in a metabolically active vascular bed and reaches an oxygen saturation of 60% at pH 7.0 and 37°C, the bimolecular rate of nitrite reduction to NO would be at 4.4 M–1s–1 and the 300 nM nitrite would react with the 12 mM intracellular deoxyheme to form 10.6 nM NO per second, a kinetic result consistent with our prior physiological observations (11). While we have shown that injections of nitrite into solutions of red cell and Hb solutions generates NO gas, additional studies are required to evaluate how NO can escape the erythrocyte and avoid autocapture by intracellular Hb (discussed more fully in Discussion).
Discussion
Physiological implications for hypoxic vasodilation.
Hypoxic vasodilation requires oxygen sensing and the rapid elaboration of a vasodilating mediator that modulates the microvascular resistance of arterioles. Consistent with an oxygen-sensor set point around Hb P50, classical physiological studies of hypoxic vasodilation consistently reveal a threshold for this response of around 40–60% Hb-oxygen saturation (PO2 of 20–40 mm Hg) (4, 22). Accumulating data obtained from measurements of microvascular longitudinal oxygen and Hb-oxygen saturation gradients suggest that arteriolar oxygen tension decreases as blood flows through A1 to A4 caliber arterioles. This drop is substantial in skeletal muscle, resulting in A4 level arteriolar PO2 values approaching 20 mm Hg and Hb saturations of less than 40% (recently reviewed in ref. 41). These decreases in oxygen have been attributed to direct oxygen delivery from arterioles, oxygen consumption by endothelium and smooth muscle, and counter-current oxygen exchange with the venous system. The latter has been reported to deliver CO2 to arterioles, an effect that lowers the Hb-oxygen affinity via the Bohr effect. These oxygen delivery profiles indicate that Hb desaturates below 50% within the skeletal muscle resistance microvasculature, a process that would also maximize Hb’s nitrite reductase activity within A4 arterioles. In the heart and brain, where most of the oxygen is delivered from the capillary bed, a retrograde propagation of the vasodilator signal has been invoked to explain feedback vasodilation from the hypoxic tissue to resistance arterioles (42, 43).
We recognize that it is likely that hypoxic vasodilation, like endothelium-dependent blood flow regulation, is overbuilt, with multiple overlapping mechanisms that ensure adequate oxygen delivery to respiring tissues. Erythrocyte- and Hb-dependent nitrite reduction to NO (11) and ATP (6–8) release would provide vasodilation at oxygen tensions from 40 to 15 mm Hg. Additional mechanisms for hypoxic vasodilation likely overlap with the erythrocyte systems, including production of adenosine, prostaglandins, NO, and endothelium-derived hyperpolarizing factor from tissue, as well as sympathetic feedforward vasodilation (recently reviewed in ref. 4).
Below 10 mm Hg oxygen tension, direct nitrite reduction to NO by nonenzymatic disproportionation (acidic reduction similar to that reported in the stomach) (44–46) and enzymatic reduction by xanthine oxidoreductase (47–49) may contribute to hypoxic vasodilation and anaerobic NO formation. Additionally, the “burst” NO formation observed for Mb (Figure 4A) is of particular interest and suggests that decreases in PO2 to below 2–4 mm Hg, as occurs during exercise, in ischemia, and in highly metabolic tissue, would be associated with a rapid conversion of tissue nitrite (approaching levels of 20 μM; ref. 37) to NO. This nitrite reductase activity of Mb (or cytoglobin and neuroglobin) might participate in the modulation of tissue mitochondrial respiration and limitation of ischemia-reperfusion injury during extremes of exercise or pathological vasoocclusion (50, 51).
NO export from the erythrocyte
? A major challenge for a role for nitrite-Hb reactions in the control of hypoxic vasodilation is the kinetics of NO reactions with vicinal deoxy- and oxyHb, which should limit NO diffusion out of the erythrocyte. However, our earlier studies reveal that physiological concentrations of nitrite vasodilate aortic rings only in the presence of deoxyHb or deoxygenated erythrocytes and that near physiological concentrations of nitrite result in vasodilation in the human forearm (11). Indeed, kinetic predictions would suggest that NO should not escape into the head space for gas-phase detection in the current study illustrated in Figures 3C and 5B. While the solution to this paradox is not yet clear, we recognize that biology may overcome such kinetic constraints. For example, a membrane-associated nitrite reductase metabolon with deoxyHb and metHb, anion exchange protein, carbonic anhydrase, aquaporin, and rhesus channels could bring together nitrite, proton, deoxyheme, and highly hydrophobic channels that could serve to concentrate the lipophilic NO at the membrane complex (52). Indeed, the heme pocket distal histidine may donate a proton to the reaction, an effect analogous to the proton donation by aspartate in the bacterial nitrite reductase enzyme (53). This histidine proton donation to nitrite would serve to both position and stabilize nitrite in the heme pocket and accelerate the reaction by forming nitrous acid.
Because of the potency of NO (50% effectivev concentration [EC50] of only 1–5 nM) and because flow is proportional to the radius to the fourth power, very little NO escape is required for vasodilation. We are currently exploring other potential intermediates that may form in the nitrite deoxyHb reaction including H2NO2 (hydrated NO), HNO2–, NO22_ (described by Doyle; ref. 19), NO, ONOO–, and N2O3. N2O3 formation by reductive nitrosylation catalyzed by nitrite has recently been characterized by Fernandez and Ford (29). Many of these intermediates would be stabilized in hydrophobic channels (H2NO2, N2O3, and NO) while others would be stabilized in anion channels, such as the anion exchange protein (HNO2–, ONOO–, and NO22_). A maximal rate of NO formation near the Hb P50 may also lead to reactions with superoxide (O2–), known to form from Hb auto-oxidation at P50 (54). Diffusion limited NO-O2– reactions can generate ONOO– and N2O3, which have the potential to nitrosate low-molecular-weight thiols in the heme pocket (55).
In conclusion, these data reveal 2 novel properties of the Hb molecule: first, the nitrite reductase activity of Hb produces NO under allosteric control; and second, the rate of nitrite reduction to NO is maximal at 40–60% Hb-oxygen saturation. These 2 properties provide a biochemical mechanism that could link allosteric oxygen sensing to vasodilation. These data suggest that Hb oxygen saturation and quaternary structure rather than tissue PO2 act as oxygen sensors for hypoxic vasodilation and that the vasodilation effector is NO, generated by a nitrite reductase activity of Hb. Understanding this chemistry should allow for therapeutic targeted NO delivery to specific tissues under hypoxic stress using nitrite solutions.
Methods
Reagents and Hb preparations.
All reagents were purchased from Sigma-Aldrich, except mutant Hb, which was provided by C. Ho. Unless otherwise indicated, Hb solutions and reactions were performed using phosphate-buffered saline, pH 7.4, at room temperature. Isolated β chains of HbA (tetramer) were prepared by C. Ho. Normal human adult Hb was prepared by hypotonically lysing washed erythrocytes, discarding the membrane fractions, and storing the hemolysate at –80°C as previously described (56). The Hb was either dialyzed or passed through a size exclusion column washed with buffer corresponding to the particular experiment. NEM-modified HbA was prepared by incubating 200-μM HbA with 10-mM NEM for 1 hour in PBS buffer at pH 7.4 in equilibrium with room air and room temperature. For IHP-bound Hb, the pH of IHP solution was adjusted to 7.4 prior to addition to deoxyHb in a 5 to 1 ratio. Excess NEM or IHP was removed by sephadex G25 column (Amersham Biosciences). The oxygen saturation of the Hb solution was measured by visible absorption spectroscopy (HP8453 UV-Vis spectrophotometer; Hewlett-Packard). The concentration and percentage of each heme species was analyzed by deconvoluting the spectrum into components from basis spectra of Hb composed of oxyHb, deoxyHb, iron-nitrosyl-Hb, carboxyHb, metHb and metHb-nitrite, using a least squares method similar to that previously described (55). CarboxyHb was included in the analysis only when carboxyHb was added to the reaction mixture. Both metHb and metHb-nitrite have significant absorption at 630 nm, and their sum was reported as the total amount of metHb or ferric hemes.
Simultaneous measurement of the nitrite reaction with Hb by visible absorption spectroscopy and NO gas detection by chemiluminescence.
In these experiments, 50-μM deoxygenated Mb, Hb, and Hb preparations partially ligated with carbon monoxide were reacted with excess nitrite (10 mM, 2.5 mM with Mb) anaerobically in a 25-ml flask for NO measurement. During this time, constant stirring and head-space purging with helium were performed (note that the solution is not purged; only the head space gas is entrained). The purged gas traveled in-line through the head space of the flask containing 5 ml of reaction mixture into a chemiluminescent NO gas analyzer (Sievers NO analyzer; GE Analytical Instruments) for detection of NO. The reaction was monitored simultaneously in a 1-cm pathlength glass cuvette under positive helium pressure for spectral measurement by visible absorbance spectrophotometry at room temperature using an HP 8953 UV-Vis spectrophotometer (Hewlett-Packard). Reaction progress was monitored by the change of absorption at 630 nm. Concentrations in deoxyheme, oxyheme, iron-nitrosyl-heme, and metheme at any time point were derived through spectral deconvolution. The instantaneous reaction rate was measured as the instantaneous rate of deoxyheme consumption (negative change of deoxyheme concentration with respect to time interval). Bimolecular rate (or bimolecular rate constant in the case of Mb) of the reaction between deoxyheme and nitrite was obtained by the instantaneous reaction rate divided by the concentration of nitrite and deoxyheme at that instant. Note that all of the UV-vis experiments were performed in anaerobic cuvettes without purging. We applied positive helium pressure without a channel for gas escape, as this prevents oxygen leaks into the system. Only the NO gas formation experiments are performed with head space gas collection (Figures 3C and 5B).
EPR spectroscopy with spectral deconvolution.
EPR spectroscopy was conducted at temperatures of 132 K with a Bruker 4111 VT variable temperature unit and ESR-ER-200 D spectrometer set (Bruker BioSpin GmbH) at 9.43 GHz microwave frequency, 10 mW microwave power, 5 Gauss modulation amplitude, a time constant of 100 ms, and a collection time of 100 seconds for each scan. The EPR spectrum for iron-nitrosyl-Hb was deconvoluted to obtain the percentage of each species of nitrosyl-heme (penta- and hexacoordinate α heme and hexacoordinate β-nitrosyl-heme) using basis spectra as described previously (26).
Acknowledgments
We acknowledge the careful work of Vidhya Annavajjhala, Catherine E. Cushenberry, and Peter Grant in support of this project. This work was supported by the Intramural Division of the NIH and partially supported by NIH grants HL58091 (to D. Kim-Shapiro) and GM55792 (to N. Hogg). EPR spectrometry was facilitated by a grant from the North Carolina Biotechnology Center (2003-IDG-1013 to D. Kim-Shapiro).
Footnotes
Nonstandard abbreviations used: E1/2, reduction (or redox) potential; EPR, electron paramagnetic resonance; Hb, hemoglobin; HbA, hemoglobin A; IHP, inositol hexaphosphate; Mb, myoglobin; NEM, N-ethylmaleimide; PO2, partial pressure of oxygen.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Gremels H, Starling EH. On the influence of hydrogen ion concentration and of anoxaemia upon the heart volume. J. Physiol. (Lond). 1926;61:297–304. doi: 10.1113/jphysiol.1926.sp002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilton R, Eichholtz F. The influence of chemical factors on the coronary circulation. J. Physiol. (Lond). 1925;59:413–425. doi: 10.1113/jphysiol.1925.sp002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 4.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J. Appl. Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 5.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence [review]? Acta Physiol. Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am. J. Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 8.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am. J. Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 9.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 10.Stamler JS, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 11.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 12.Hunter CJ, et al. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 13.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;273:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 14.Huang, K.T., et al. 2005. The reaction between nitrite and deoxyhemoglobin: reassessment of reaction kinetics and stoichiometry. J. Biol. Chem. doi:10.1074/jbc.M501496200. [DOI] [PubMed]
- 15.Ignarro LJ, Gruetter CA. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim. Biophys. Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J. Pharmacol. Exp. Ther. 1953;108:129–143. [PubMed] [Google Scholar]
- 17.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U. S. A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladwin MT, et al. Relative role of heme nitrosylation and beta -cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9943–9948. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 20.Brooks J. The action of nitrite on haemoglobin in the absence of oxygen. Proc. R. Soc. Med. 1937;123:368–382. [Google Scholar]
- 21.Hsia CC. Respiratory function of hemoglobin. N. Engl. J. Med. 1998;338:239–247. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 22.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am. J. Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 24.Yonetani T, Tsuneshige A, Zhou Y, Chen X. Electron paramagnetic resonance and oxygen binding studies of alpha- nitrosyl hemoglobin. A novel oxygen carrier having no-assisted allosteric functions. J. Biol. Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 25.Hille R, Olson JS, Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J. Biol. Chem. 1979;254:12110–12120. [PubMed] [Google Scholar]
- 26.Xu X, et al. Effects of iron nitrosylation on sickle cell hemoglobin solubility. J. Biol. Chem. 2002;277:36787–36792. doi: 10.1074/jbc.M205350200. [DOI] [PubMed] [Google Scholar]
- 27.Taboy CH, Faulkner KM, Kraiter D, Bonaventura C, Crumbliss AL. Concentration-dependent effects of anions on the anaerobic oxidation of hemoglobin and myoglobin. J. Biol. Chem. 2000;275:39048–39054. doi: 10.1074/jbc.M004547200. [DOI] [PubMed] [Google Scholar]
- 28.Bonaventura C, et al. Heme redox properties of S-nitrosated hemoglobin A0 and hemoglobin S: implications for interactions of nitric oxide with normal and sickle red blood cells. J. Biol. Chem. 2002;277:14557–14563. doi: 10.1074/jbc.M107658200. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez BO, Lorkovic IM, Ford PC. Nitrite catalyzes reductive nitrosylation of the water-soluble Ferri-Heme model Fe(III)(TPPS) to Fe(II)(TPPS)(NO) Inorg. Chem. 2003;42:2–4. doi: 10.1021/ic020519r. [DOI] [PubMed] [Google Scholar]
- 30.Luchsinger BP, et al. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J. Inorg. Biochem. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Bohr C, Hasselbalch K, Krogh A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensaeurespannung des Blutes auf dessen Sauerstoffbindung ubt. Skand. Arch. Physiol. 1904;16:402–412. [Google Scholar]
- 32.Perutz MF. Sterochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman BM, Bull C. Linearity of the hemoglobin oxidation bohr effect. Proc. Natl. Acad. Sci. U. S. A. 1976;73:800–803. doi: 10.1073/pnas.73.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunori M, Wyman J, Antonini E, Rossi-Fanelli A. Studies on the oxidation-reduction potentials of heme proteins. V. The oxidation Bohr effect in normal human hemoglobin and human hemoglobin digested with carboxypeptidase A. J. Biol. Chem. 1965;240:3317–3324. [PubMed] [Google Scholar]
- 35.Antonini E, et al. Studies on the oxidation-reduction potentials of heme proteins. I. Human hemoglobin. J. Biol. Chem. 1964;239:907–912. [PubMed] [Google Scholar]
- 36.Taylor JF, Hastings AB. Oxidation-reduction potentials of the methemoglobin-hemoglobin system. J. Biol. Chem. 1939;131:649–662. [Google Scholar]
- 37.Dejam, A., et al. 2005. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. doi:10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed]
- 38.Bryan NS, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen FB, Andersen NA, Heisler N. Effects of nitrite exposure on blood respiratory properties, acid-base and electrolyte regulation in the carp (Cyprinus carpio) J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 1987;157:533–541. doi: 10.1007/BF00700972. [DOI] [PubMed] [Google Scholar]
- 40.Jensen FB, Koldkjaer P, Bach A. Anion uptake and acid-base and ionic effects during isolated and combined exposure to hypercapnia and nitrite in the freshwater crayfish, Astacus astacus. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 2000;170:489–495. doi: 10.1007/s003600000126. [DOI] [PubMed] [Google Scholar]
- 41.Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol. Rev. 2003;83:933–963. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- 42.Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am. J. Physiol. 1989;256:H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- 43.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am. J. Physiol. 1989;256:H838–H845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health [review] [erratum 2004, 2:681] Nat. Rev. Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 45.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 46.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. . Biochim. Biophys. Acta. 1999; 1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, et al. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity [erratum 1998, 251:667] Biochem. Biophys. Res. Commun. 1998;249:767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- 48.Millar TM, et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J. Biol. Chem. 2004;279:16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 50.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi:10.1172/JCI200522493. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation [review] Free Radic. Biol. Med. 2004;36:707–717. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 53.Tocheva EI, Rosell FI, Mauk AG, Murphy ME. Side-on copper-nitrosyl coordination by nitrite reductase. Science. 2004;304:867–870. doi: 10.1126/science.1095109. [DOI] [PubMed] [Google Scholar]
- 54.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 55.Espey MG, Thomas DD, Miranda KM, Wink DA. Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11127–11132. doi: 10.1073/pnas.152157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Z, et al. Nitric oxide binding to oxygenated hemoglobin under physiological conditions. . Biochim. Biophys. Acta. 2001; 1568:252–260. doi: 10.1016/s0304-4165(01)00227-6. [DOI] [PubMed] [Google Scholar]