Abstract
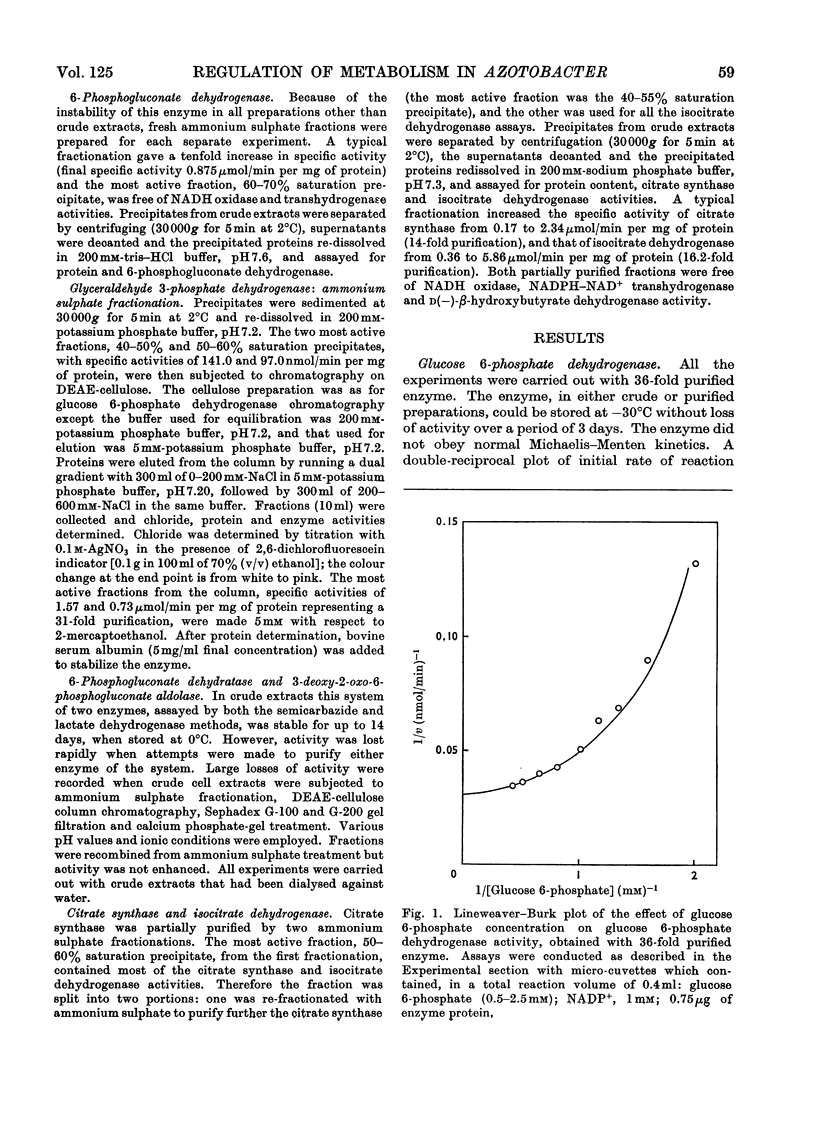
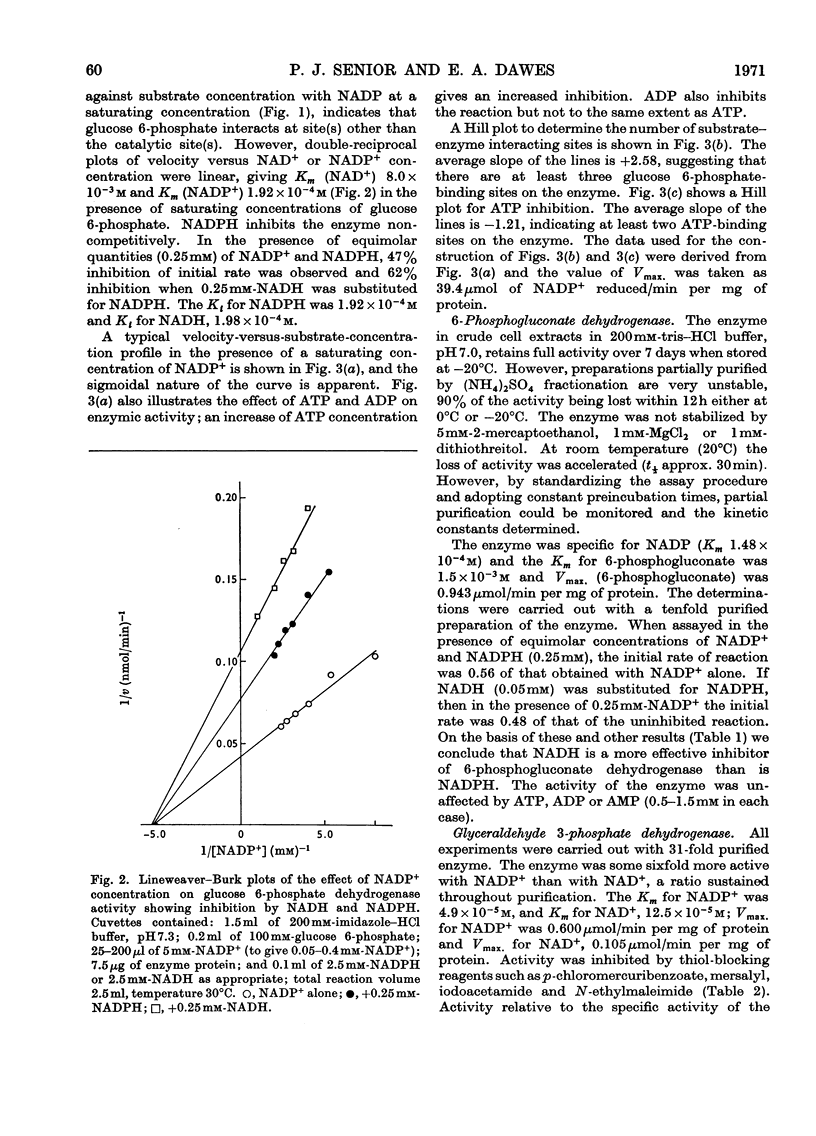
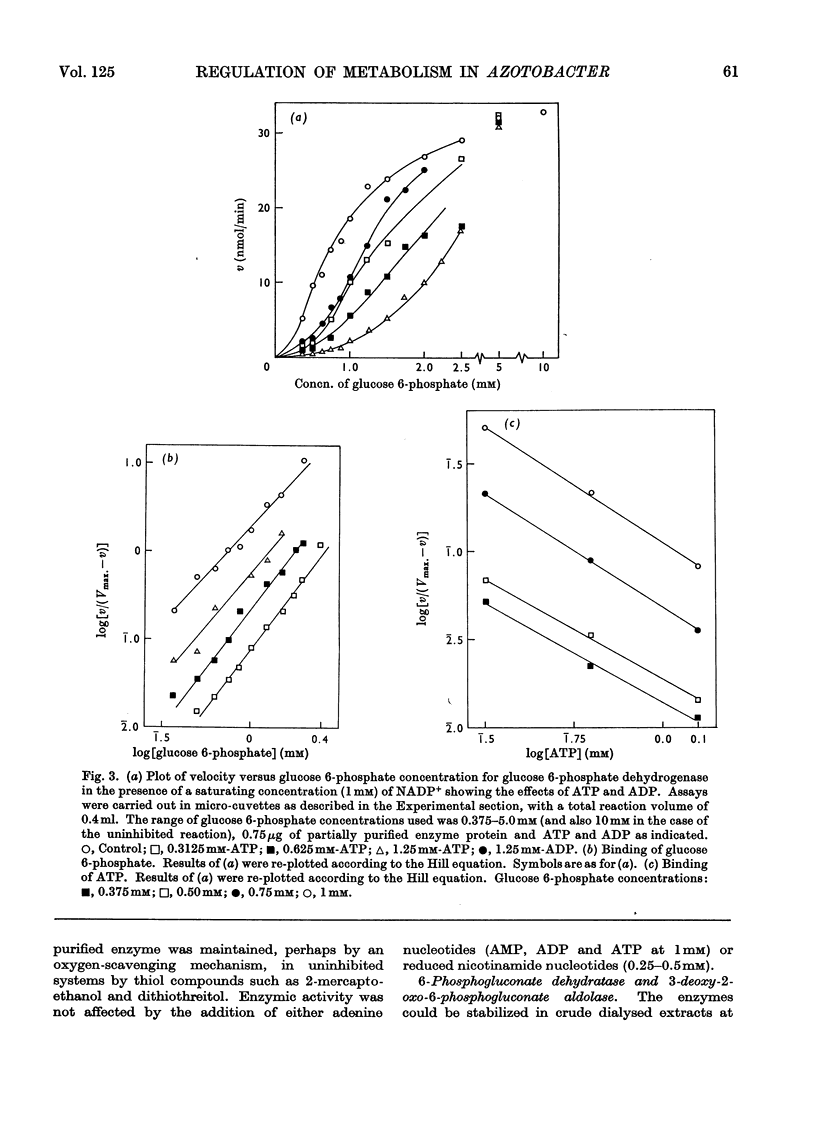
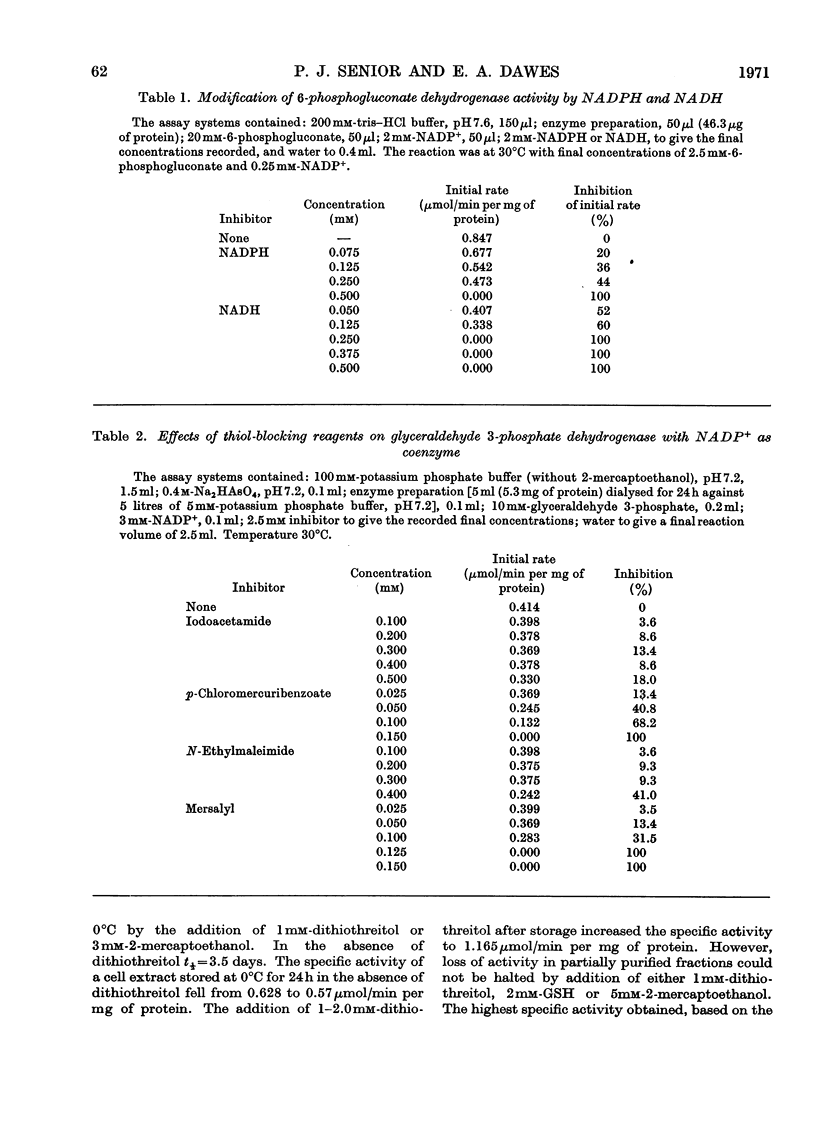
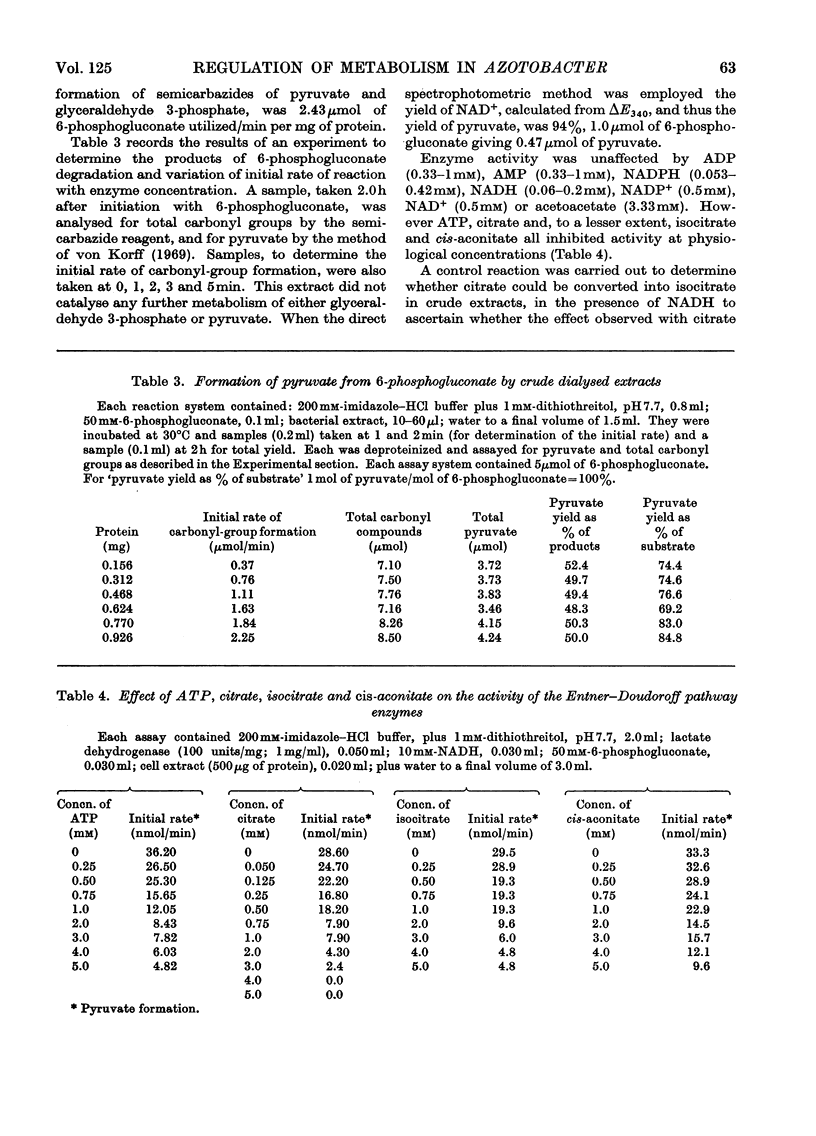
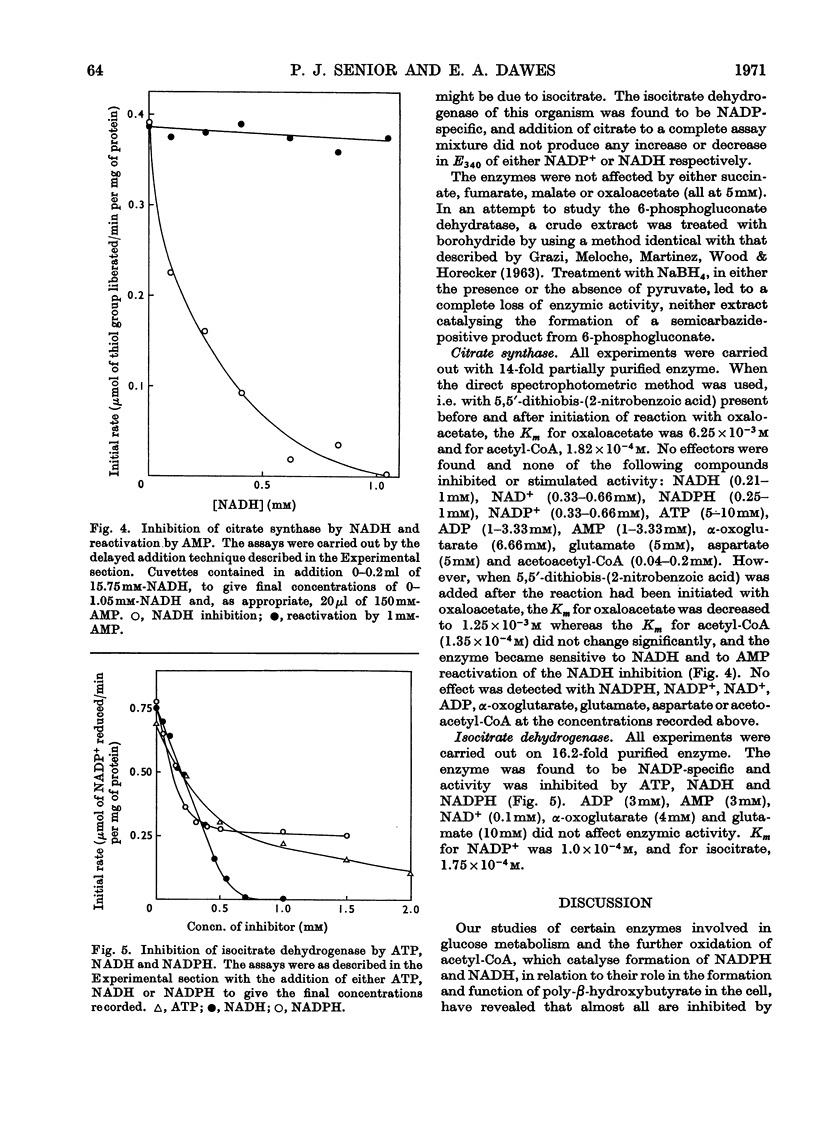
Azotobacter beijerinckii possesses the enzymes of both the Entner–Doudoroff and the oxidative pentose phosphate cycle pathways of glucose catabolism and both pathways are subject to feedback inhibition by products of glucose oxidation. The allosteric glucose 6-phosphate dehydrogenase utilizes both NADP+ and NAD+ as electron acceptors and is inhibited by ATP, ADP, NADH and NADPH. 6-Phosphogluconate dehydrogenase (NADP-specific) is unaffected by adenosine nucleotides but is strongly inhibited by NADH and NADPH. The formation of pyruvate and glyceraldehyde 3-phosphate from 6-phosphogluconate by the action of the Entner–Doudoroff enzymes is inhibited by ATP, citrate, isocitrate and cis-aconitate. Glyceraldehyde 3-phosphate dehydrogenase is unaffected by adenosine and nicotinamide nucleotides but the enzyme is non-specific with respect to NADP and NAD. Citrate synthase is strongly inhibited by NADH and the inhibition is reversed by the addition of AMP. Isocitrate dehydrogenase, a highly active NADP-specific enzyme, is inhibited by NADPH, NADH, ATP and by high concentrations of NADP+. These findings are discussed in relation to the massive synthesis of poly-β-hydroxybutyrate that occurs under certain nutritional conditions. We propose that synthesis of this reserve material, to the extent of 70% of the dry weight of the organism, serves as an electron and carbon `sink' when conditions prevail that would otherwise inhibit nitrogen fixation and growth.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Jurtshuk P. Characterization of the highly active isocitrate (NADP+) dehydrogenase of Azotobacter vinelandii. Biochim Biophys Acta. 1970 Dec 16;220(3):416–429. doi: 10.1016/0005-2744(70)90273-1. [DOI] [PubMed] [Google Scholar]
- Chung A. E. Pyridine nucleotide transhydrogenase from Azotobacter vinelandii. J Bacteriol. 1970 May;102(2):438–447. doi: 10.1128/jb.102.2.438-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAZI E., MELOCHE H., MARTINEZ G., WOOD W. A., HORECKER B. L. Evidence for Schiff base formation in enzymatic aldol condensations. Biochem Biophys Res Commun. 1963 Jan 18;10:4–10. doi: 10.1016/0006-291x(63)90257-2. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. J., JOHNSON M. K. Alternate pathwayy of glucose metabolism in Azotobacter agilis. Proc Soc Exp Biol Med. 1961 Dec;108:728–731. doi: 10.3181/00379727-108-27048. [DOI] [PubMed] [Google Scholar]
- Kersters K., De Ley J. An easy screening assay for the enzymes of the Entner-Doudoroff pathway. Antonie Van Leeuwenhoek. 1968;34(4):388–392. doi: 10.1007/BF02046461. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., WILSON P. W. Initial stages in the breakdown of carbohydrates by the Azotobacter vinelandii. Arch Biochem Biophys. 1954 Dec;53(2):425–435. doi: 10.1016/0003-9861(54)90423-3. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., WILSON P. W. Metabolism of ribose-5-phosphate by Azotobacter vinelandii. J Biol Chem. 1955 Apr;213(2):713–721. [PubMed] [Google Scholar]
- Pearce J., Carr N. G. The incorporation and metabolism of glucose by Anabaena variabilis. J Gen Microbiol. 1968 Dec;54(3):451–462. doi: 10.1099/00221287-54-3-451. [DOI] [PubMed] [Google Scholar]
- Ritchie G. A., Dawes E. A. The non-involvement of cyl-carrir protein in poly-beta-hydroxybutyric acid biosynthesis in Azotobacter beijerinckii. Biochem J. 1969 May;112(5):803–805. doi: 10.1042/bj1120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Senior P. J., Dawes E. A. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J. 1971 Jan;121(2):309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J., Schlegel H. G. Regulation der Glucose-6-phosphat-Dehydrogenase aus verschiedenen Bakterienarten durch ATP. Arch Mikrobiol. 1969;66(1):69–78. [PubMed] [Google Scholar]
- Senior P. J., Dawes E. A. The glyceraldehyde 3-phosphate dehydrogenase of Azotobacter beijerinckii and its possible significance in poly-beta-hydroxybutyrate biosynthesis. Biochem J. 1970 Oct;119(5):38P–38P. doi: 10.1042/bj1190038pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman P. D., Jones D. Regulation of citrate synthase and microbial taxonomy. Nature. 1968 Jul 20;219(5151):270–272. doi: 10.1038/219270a0. [DOI] [PubMed] [Google Scholar]


