Abstract
Background
Wnt signaling is implicated in many developmental decisions, including stem cell control, as well as in cancer. There are relatively few target genes known of the Wnt pathway.
Results
We have identified target genes of Wnt signaling using microarray technology and human embryonic carcinoma cells stimulated with active Wnt protein. The ~50 genes upregulated early after Wnt addition include the previously known Wnt targets Cyclin D1, MYC, ID2 and βTRCP. The newly identified targets, which include MSX1, MSX2, Nucleophosmin, Follistatin, TLE/Groucho, Ubc4/5E2, CBP/P300, Frizzled and REST/NRSF, have important implications for understanding the roles of Wnts in development and cancer. The protein synthesis inhibitor cycloheximide blocks induction by Wnt, consistent with a requirement for newly synthesized β-catenin protein prior to target gene activation. The promoters of nearly all the target genes we identified have putative TCF binding sites, and we show that the TCF binding site is required for induction of Follistatin. Several of the target genes have a cooperative response to a combination of Wnt and BMP.
Conclusions
Wnt signaling activates genes that promote stem cell fate and inhibit cellular differentiation and regulates a remarkable number of genes involved in its own signaling system.
Background
There is currently great interest in using extracellular signaling proteins to influence the gene expression program and differentiation of embryonic cells, in particular embryonic stem cells. Of the factors that contribute to specifying cell fate during development, Wnt proteins are among the most attractive candidates to use in such in vitro experiments. Wnt proteins control numerous aspects of development, ranging from stem cell control to differentiation and cell polarity [1,2]. It has been problematic however to test directly whether Wnt proteins can be used as reagents in cell culture, because working with soluble Wnt proteins is difficult and few cell lines are known to respond to Wnt proteins. Wnt signal transduction proceeds through a complex series of protein interactions, initiated by binding of the Wnt protein to cell surface receptors (Frizzled and LRP [3-5]) which generates a signal to downstream components. A key event in signaling is the regulation of the GSK3 protein kinase and its substrate β-catenin. In the absence of a Wnt signal, GSK3 phosphorylates β-catenin, which then becomes targeted for degradation [6]. The binding of Wnt to its receptors initiates a cascade of events that inhibit GSK3 and ultimately prevent degradation of β-catenin. Together with the DNA binding protein TCF, β-catenin activates expression of Wnt target genes. In this work, we have identified Wnt target genes using micro-array technology.
Results
Identifying Wnt targets on microarrays
We tested human teratocarcinoma cells (NCCIT) cells for a Wnt response. Despite the cancerous origin of these cells, they share many properties with embryonic stem cells [7]. These cells express several members of the Frizzled family (including FZD7), one of the receptors for Wnt (data not shown; Figure 2A). To stimulate the NCCIT cells with Wnt, we used tissue culture medium containing active Wnt-3A protein produced by mouse L cells (Wnt-3A CM [8]), in comparison to control conditioned medium (CCM). In initial experiments, we found that Wnt-3A protein elevates the levels of β-catenin 5–10 fold (Figure 4); and that a transiently transfected TCF reporter construct is activated 3–4 fold (not shown).
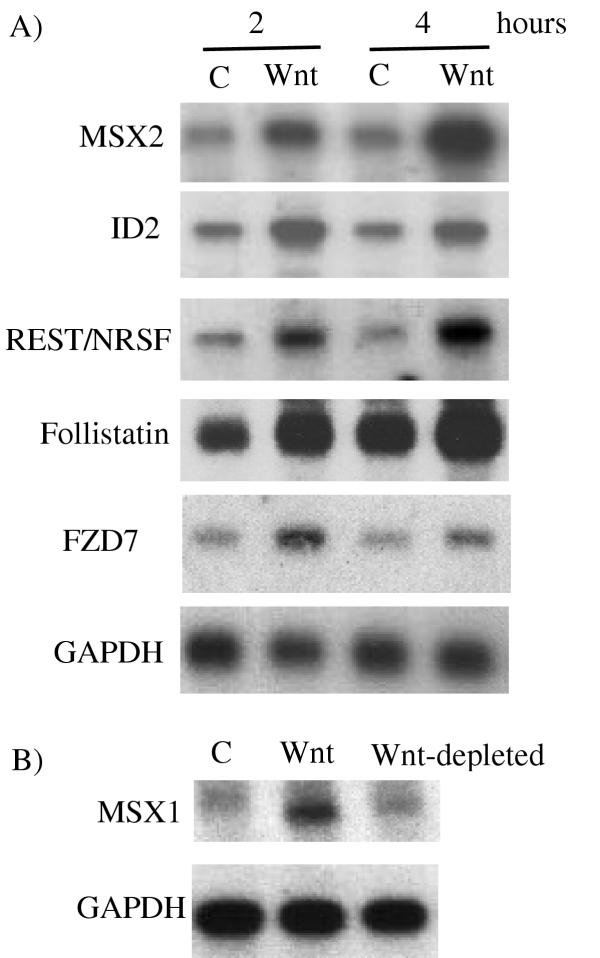
Figure 2.
A) Confirmation of microarray data using Northern analysis. Northern blot analysis using MSX2, ID2, REST/NRSF, FZD7 (Frizzled7) and Follistatin cDNA probes. NCCIT cells were treated with CCM (-) or Wnt-3A CM (+) for the designated number of hours. 1 ug mRNA was loaded into each lane. GAPDH is shown as a sample loading control. B) MSX1 induction by Wnt-3A CM is dependent on the presence of soluble Wnt-3A protein. C = CCM. Wnt = Wnt-3A CM. Wnt-depleted = Wnt-3A CM. Cells were incubated with the various media for 4 hours.
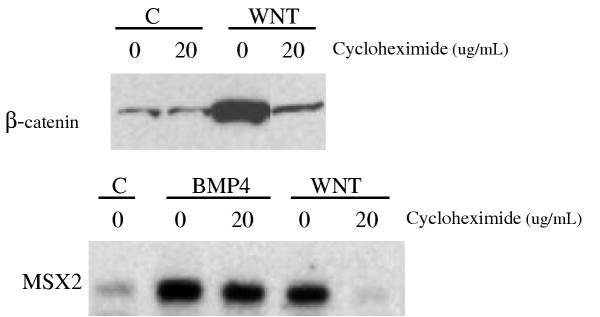
Figure 4.
A) Western blot demonstrating cycloheximide effects of Wnt-3A mediated accumulation of β-catenin. NCCIT cells were stimulated for 4 hours with CCM (C) or Wnt-3A CM (Wnt) in the presence (20 microgram/ml) or absence (0) of cycloheximide. Note that β-catenin does not accumulate in response to Wnt-3A CM when cycloheximide is present. B) Northern analysis demonstrating that MSX2 induction by Wnt-3A is abolished in the presence of cycloheximide. Cells were stimulated for 4 hours with CCM (C), BMP-4 or Wnt-3A CM (Wnt) in the presence (20 microgram/ml media) or absence of cycloheximide. As expected, BMP-4 mediated induction of MSX2 is not affected by cycloheximide which contrasts with the inhibition of Wnt-3A mediated induction of MSX2 in the presence of cycloheximide. GAPDH was used to verify equal loading. The inhibitory effect was also seen for MSX1, ID2, Follistatin, Versican and Cyclin D1 (not shown).
To identify target genes, we applied Wnt-3A CM or CCM to the NCCIT cells and isolated RNA at several time points. We performed differential hybridization to microarray slides containing approximately 23,000 spots of human cDNAs, using RNA from CCM-exposed cells as a reference. Figure 1 shows a cluster analysis of the hybridization results. We found approximately 50 genes that were upregulated between 2 and 10 fold by Wnt-3A CM whereas a few genes were repressed, i.e. expressed at lower levels in the Wnt-3A-treated cells. The latter group consisted mostly of ESTs of unknown genes and has yet to be characterized.
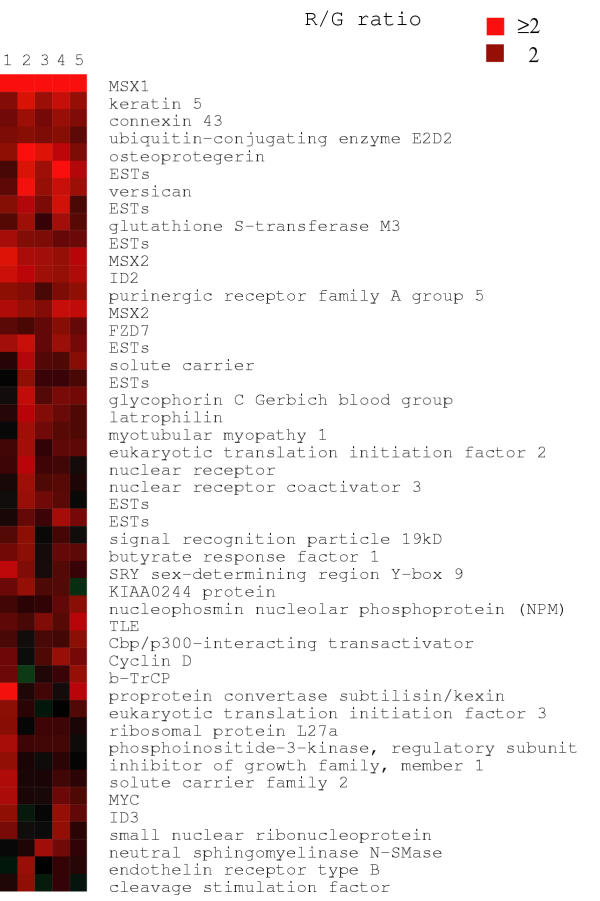
Figure 1.
Microarray cluster analysis demonstrating increased expression of Wnt-3A responsive genes, including MSX1, MSX2, ID2, Versican, NPM, Frizzled-7, TLE/Groucho, Cyclin D1, and MYC. NCCIT cells were exposed to control conditioned medium (CCM) and Wnt-3A CM [8] for 4 hours. Shown here are 5 separate experiments. Note also that MSX 2 is included on this particular array in duplicate which provides an internal check on the precision of the measurements. Additional array experiments led to the identification of Follistatin and REST/NRSF as elevated genes (which were confirmed in Figure 2A). Brightest red corresponds to ratio of = 2:1 and dimmer red corresponds to ratio of 2:1. The full data set will be available at the Wnt homepage http://www.stanford.edu/~rnusse/wntwindow.html and the Stanford Microarray database http://genome-www5.stanford.edu/MicroArray/SMD/
We verified Wnt-induced expression of several genes with Northern blots (Figure 2). In all cases tested, we confirmed the increase observed in the microarray; in some cases we found even higher differences in expression. For example, MSX2, ID2, REST/NRSF, FZD7 and Follistatin were confirmed by Northern analysis to be significantly elevated by Wnt-3A CM after two hours (Figure 2A).
None of these genes was induced by the CCM, suggesting that they are Wnt-specific. It remained possible however that the observed changes in gene expression were influenced by other differences between Wnt-3A CM and CCM. For example, Wnt-3A could have induced the expression of another secreted factor in the mouse L cells which would then be present only in the Wnt-3A CM. We addressed this possibility by specifically removing active Wnt-3A from the conditioned medium, using a combination of the ligand binding domain of the Drosophila Wnt/Wingless receptor Frizzled-2 fused to Alkaline Phosphatase [3,9] and Wnt-3A specific antibodies. This procedure specifically depleted active Wnt-3A from the medium. As expected, neither β-catenin levels nor a TCF reporter gene were elevated by the depleted CM (not shown). The depleted medium also failed to induce MSX1, MSX2, ID2 and CyclinD1 expression as assayed by Northern blots (Figure 2B shows loss of MSX1) and in a DNA microarray analysis with CCM as a reference (not shown). Similarly, adding Dickkopf protein, an inhibitor of Wnt signaling that binds to the co-receptor LRP [10], to the medium abrogated the transcriptional response (data not shown). We conclude that the transcriptional response to Wnt-3A CM is indeed controlled by the Wnt-3A protein rather than by other factors.
Cooperativity between Wnt-3A and BMP
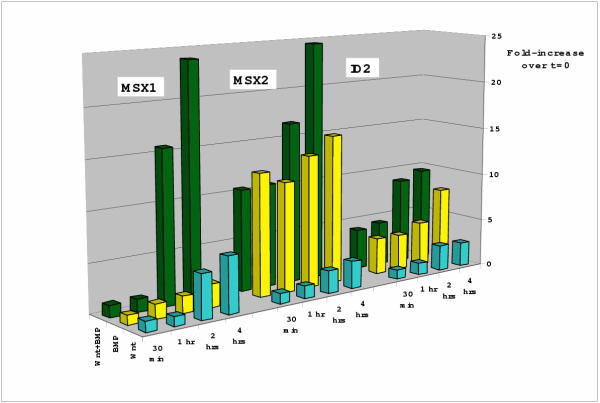
Several of the Wnt target genes had been identified previously as targets of BMP signaling [11]. We were therefore interested in comparing the effects of BMP and Wnt-3A and examining their combined effects. As has been reported before for other cells [11], MSX1, MSX2 and ID2 are elevated by BMP-4 in NCCIT cells (Figure 3). When Wnt-3A and BMP-4 were combined, gene expression was increased to yet higher levels. MSX1 expression was markedly elevated by the combination of BMP-4 and Wnt-3A. Similar but less dramatic effects were found for ID2 and MSX2 (Figure 3). Hence, BMP-4 and Wnt-3A appear to have an additive or perhaps synergistic effect on target gene expression. However, when the time courses of Wnt- and BMP-induced gene expression were compared, it appeared that BMP-4 acts as soon as at 30 minutes, but that Wnt takes 2 hours to have an effect (Figure 3). Hence, Wnt-induced changes in gene expression are slower than BMP, even on the same target genes and in the same cell type.
Figure 3.
Time course of Wnt induced gene expression and cooperation with BMP. NCCIT cells were exposed to Wnt-3A CM, BMP-4 (10 ng/ml final concentration) or the combination of Wnt-3A and BMP-4 for the specified number of hours. Shown here are MSX1, MSX2 and ID2 demonstrating that Wnt-3A induced effects are not seen until 2 hours (which corresponds to β-catenin accumulation). BMP-4 mediated induction of MSX1, MSX2 and ID2 occurs rapidly, as early as 30 minutes and stays relatively constant. Cooperative effects of Wnt-3A and BMP-4 are not evident until approximately 2 hours. The height of the bars presents the ratio induced/non-induced, quantified by phosphoimager analysis of Northern blots and normalized to GAPDH expression measured in the same experiment. Each bar represents average values obtained from 3–5 experiments for each mRNA tested.
Wnt signaling is blocked by cycloheximide
The delay in transcriptional response to soluble Wnt raised the concern that the genes we identified might not be primary targets of Wnt but dependent on the prior induction of other genes. Such concerns are usually addressed by blocking protein synthesis in cells because the expression of primary target genes, or so-called immediate early genes, should not be sensitive whereas secondary targets should be blocked. This approach is not readily applicable to the Wnt response because the critical step in signal transduction is the block in β-catenin phosphorylation and degradation, leading to the accumulation of newly-synthesized β-catenin. Whether Wnt target gene activation is cycloheximide sensitive has never been tested however. When we incubated cells with cycloheximide, the accumulation of β-catenin by Wnt-3A was blocked; and Wnt targets, like MSX2, were not elevated (Figure 4). The inhibitory effect was also seen for MSX1, ID2, Follistatin, Versican and Cyclin D1 (not shown). In contrast, cycloheximide did not block induction of target genes by BMP (Figure 4), the mechanism of which does not involve protein accumulation [12]. These findings underscore the importance of β-catenin protein elevation in the response to Wnt. This unique aspect of Wnt signaling may explain its relatively slow effects on target genes – protein synthesis is slower than protein phosphorylation or other signal transduction events.
The requirement for protein synthesis in Wnt signaling means that we cannot use cycloheximide sensitivity as a criterion to discriminate between primary and secondary targets.
The Follistatin promoter is dependent on a TCF binding site
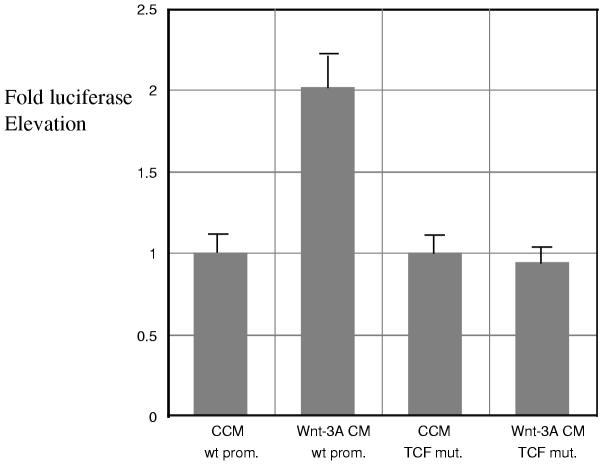
In the Wnt target gene promoters that are functionally mapped (MSX1, MSX2, ID2, ID3, Follistatin, REST/NRSF, Versican) we invariably found TCF binding sites. Moreover, we found that a luciferase reporter gene, placed under the control of the Follistatin promoter, was activated by Wnt-3A protein added to transiently transfected NCCIT cells (Figure 5). Mutating the single TCF binding site on the Follistatin promoter eliminated the response. These data suggest that Follistatin is activated directly through the Wnt signal transduction pathway and not by another pathway. Because the kinetics of Follistatin activation by Wnt are similar to that of the other target genes (Figure 2A), we suggest that there are no "earlier" targets, i.e. all identified targets are direct.
Figure 5.
Response of a reporter containing the Follistatin promoter to Wnt-3A protein in NCCIT cells [37]. The Follistatin promoter [36] contains one putative TCF binding site (CTTTGAT). This promoter linked to luciferase was transfected in NCCIT cells, which were then exposed to Wnt-3A CM and CCM for 8 hours. This assay showed a significant increase in activity when cells were stimulated directly with Wnt-3A CM or co-transfected with activated β-catenin (not shown). This effect was abrogated by co-transfection of dominant-negative TCF-4 or axin (not shown). When the TCF site within the Follistatin promoter was mutated into CATCGAT, the Wnt-3A response was abolished.
Discussion
The overall pattern of gene expression observed in response to Wnt has important implications for the role of Wnt signaling in development and cancer. Of particular interest are the ID, MSX, and REST/NRSF genes. Expression of each of these genes can interfere with differentiation of cells. ID2 was recently shown also to be a target of activated β-catenin in colon carcinoma cells [13]. It is a general inhibitor of the bHLH proteins such as MyoD and E12/E47 [14] and can interact with the tumor suppressor RB1 [15]. MSX1 expression can revert differentiated myotubes into an undifferentiated state [16]. REST/NRSF is known to block neural differentiation by binding to and inhibiting the expression of neuronal genes [17,18]. To what extent these genes are controlled by Wnt signaling in intact animals or in other cell types is not clear yet, but we do note that MSX mutants have phenotypes similar to LEF/TCF mutants [19,20].
The MSX and ID genes are also activated by BMP [11] and we found indeed synergistic effects of Wnt plus BMP. There are several examples of interactions between BMP and Wnt family members in vivo, particularly in Drosophila [21] and this cross-talk adds to the potential of these pathways for cell type specification. However, Wnt signaling also promotes the expression of such genes as Cyclin D1 [22] and MYC [23], which are not elevated by BMP. The ability to block differentiation and simultaneously promote cell division is a distinctive characteristic of Wnt signaling which could be instrumental in the role of Wnts as stem cell growth factors, such as in the crypt of the colon, and in carcinogenesis [2,24,25].
Several of the Wnt targets genes encode components of the Wnt signaling transduction system. The increased expression of βTrCP/slimb [26] and the ubiquitin conjugating enzyme Ubc4/5E2 (Figure 1), both involved in degradation of β-catenin [27], could act as a negative feedback loop in Wnt signaling. Moreover, Frizzled receptors, Versican and other proteoglycans which contribute to Wnt protein activity are also controlled by Wnt signaling, as previously described in Drosophila [28,29]. At the level of transcriptional regulation, we found up-regulation of CBP/P300, which partners with β-catenin [30,31] but also TLE/Groucho, which is an inhibitor of TCF function [32]. These responses suggest a rich system of homeostasis and feedback control mechanisms, but they complicate the interpretation of experimental studies of Wnt signal transduction in which the time course of induction cannot be controlled easily.
The delayed response to Wnt signaling raises interesting developmental issues. Timing of gene expression is very important during embryogenesis. A slow response is likely to be an important feature of the activity of Wnts as morphogens, when not only the concentration but also the duration of the stimulus is important. In considering these issues, it is of interest to note that in the early C. elegans embryo, Wnt signaling proceeds through a non-canonical pathway, through phosphorylation of the TCF homolog but not using the increase in β-catenin [33]. Cell divisions and polarization in the worm embryo occur on a time scale of minutes, and the different mechanism of Wnt signaling in that context may be more suitable than the inherently slower translation-dependent β-catenin increase.
Conclusions
We found that Wnt signaling can activate genes that promote stem cell fate and inhibit cellular differentiation; and regulates a remarkable number of genes involved in its own signaling system. The overall pattern of gene expression provides clues to the diverse functions of Wnt proteins in cancer, cell fate decisions and other developmental events.
Methods
Cells
Mouse L cells secreting Wnt-3A were established in our lab, by transfecting expression constructs in which the Wnt-3A cDNA [34] is driven by the PGK promoter. In order to get cells secreting relatively high amounts of Wnt-3A, we selected clones from the transfected cell population. The tissue culture supernatant was added to NCCIT cells [7].
Hybrizations and array experiments
mRNA isolated from Wnt-3A exposed cells was reverse transcribed and labeled with Cy5 (red) and cDNA from CCM treated cells labeled with Cy3 (green). Samples from induced and control NCCIT cells were mixed and co-hybridized to array slides containing 22,648 different human cDNAs, representing 17,083 different Unigene clusters and including 7,230 characterized genes. Hybridized arrays were imaged using a GenPix (Axon) scanner and data were analyzed by hierarchical clustering [35].
Depletion of Wnt protein
The medium was specifically depleted of Wnt-3A by adding an excess of a fusion protein containing the cysteine-rich domain of the Frizzled2 receptor of Drosophila (which binds to Wnt-3A protein) and alkaline phosphatase. [9]. By adding antibody to alkaline phosphatase, all of the detectable Wnt-3A protein could be removed from the medium. In addition, an antibody to Wnt-3A itself was used for depletion, although it worked less efficiently than the Frizzled fusion protein
Mutating the Follistatin promoter
The Follistatin promoter [36] contains one putative TCF binding site (CTTTGAT) at position -223 to -217 from the start of transcription. This site was mutated into CATCGAT, which led to abrogation of the Wnt-3A response.
Authors' contributions
JW , ME and JP performed the experiments. JW and ME made equal contributions to this work. PB and RN supervised the work. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Catriona Logan and Karl Willert for comments on the manuscript. The initial TCF reporter assays were done by Dr. Bo Yu. We thank Dr. S. Winters for the Follistatin promoter luciferase construct. These studies were supported by the Howard Hughes Medical Institute.
Contributor Information
Jennifer Willert, Email: jennifer.willert@cox.net.
Mirjam Epping, Email: epping_m_t_@hotmail.com.
Jonathan R Pollack, Email: pollack1@stanford.edu.
Patrick O Brown, Email: pbrown@cmgm.stanford.edu.
Roel Nusse, Email: rnusse@cmgm.stanford.edu.
References
- Cadigan K, Nusse R. Wnt signaling: a common theme in animal development. Genes & Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Harryman Samos C, Hsieh JC, Wang YS, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1002/(SICI)1096-9861(19990517)407:4<527::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Yang-Snyder JA, Miller JR. Structurally related receptors and antagonists compete for secreted Wnt ligands. Cell. 1997;88:725–8. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- Polakis P. More than one way to skin a catenin. Cell. 2001;105:563–6. doi: 10.1016/S0092-8674(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Damjanov I, Horvat B, Gibas Z. Retinoic acid-induced differentiation of the developmentally pluripotent human germ cell tumor-derived cell line, NCCIT. Lab Invest. 1993;68:220–32. [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of Axin releases b-catenin from the Axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–26. [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–45. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman SP, Currie SA, Ciavarella M, Vincan E, Dow C, Thomas RJ, Phillips WA. Id2 is a target of the beta-catenin/T cell factor pathway in colon carcinoma. J Biol Chem. 2001;276:45113–9. doi: 10.1074/jbc.M107742200. [DOI] [PubMed] [Google Scholar]
- Norton JD, Deed RW, Craggs G, Sablitzky F. Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol. 1998;8:58–65. [PubMed] [Google Scholar]
- Lasorella A, Noseda M, Beyna M, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–8. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of Mammalian Myotubes Induced by msx1. Cell. 2000;103:1099–1109. doi: 10.1016/s0092-8674(00)00212-9. [DOI] [PubMed] [Google Scholar]
- Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–57. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–3. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Thuringer F, Cohen SM, Bienz M. Dissection of an indirect autoregulatory response of a homeotic drosophila gene. EMBO J. 1993;12:2419–2430. doi: 10.1002/j.1460-2075.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–20. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor [In Process Citation]. Mol Cell. 2000;5:877–82. doi: 10.1016/s1097-2765(00)80327-5. [DOI] [PubMed] [Google Scholar]
- Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning AM, Andersen JS, Mann M, Mercurio F, Ben-Neriah Y. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396:590–4. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Sato A, Kojima T, Ui-Tei K, Miyata Y, Saigo K. Dfrizzled-3, a new Drosophila Wnt receptor, acting as an attenuator of Wingless signaling in wingless hypomorphic mutants. Development. 1999;126:4421–30. doi: 10.1242/dev.126.20.4421. [DOI] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. Embo J. 2000;19:1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–12. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–26. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- Roelink H, Nusse R. Expression of two members of the Wnt gene family during mouse development; restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanaga K, Shimasaki S. Structural and functional characterization of the rat follistatin (activin-binding protein) gene promoter. Mol Cell Endocrinol. 1993;92:99–109. doi: 10.1016/0303-7207(93)90080-4. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Dalkin AC, Tsujii T. Evidence that pituitary adenylate cyclase activating polypeptide suppresses follicle-stimulating hormone-beta messenger ribonucleic acid levels by stimulating follistatin gene transcription. Endocrinology. 1997;138:4324–9. doi: 10.1210/endo.138.10.5441. [DOI] [PubMed] [Google Scholar]