Abstract
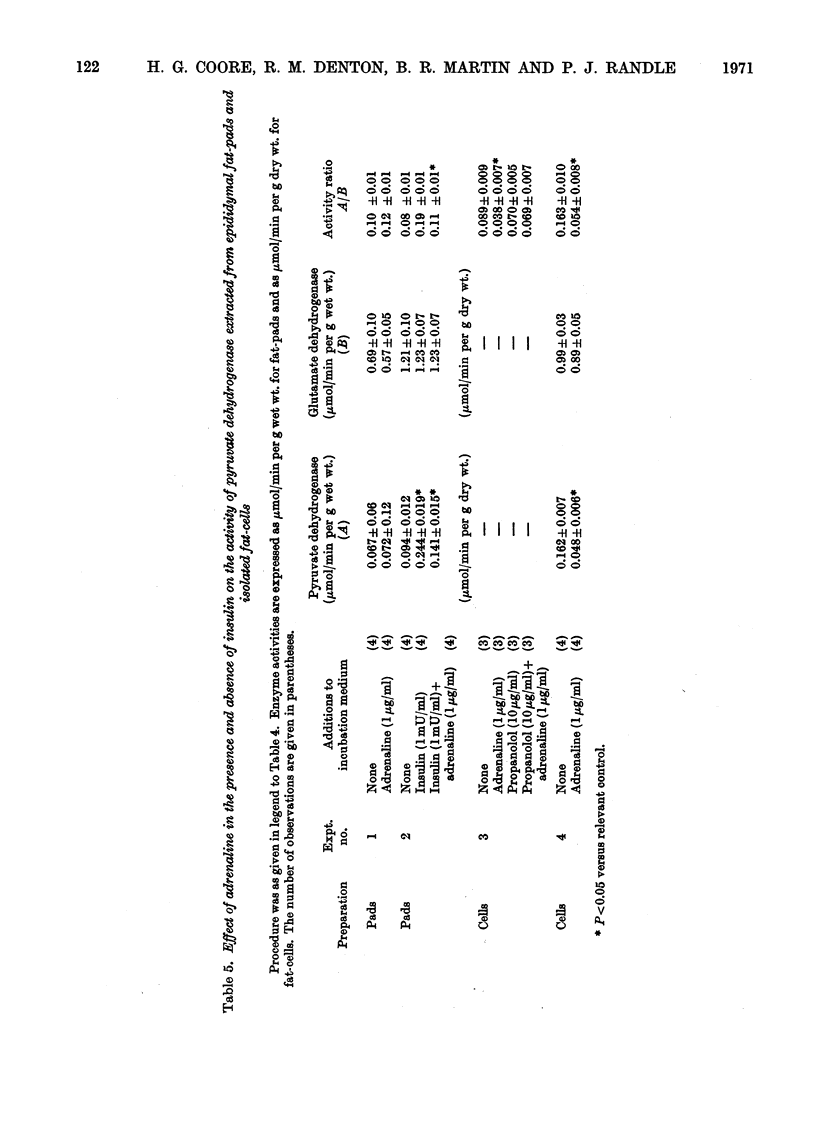
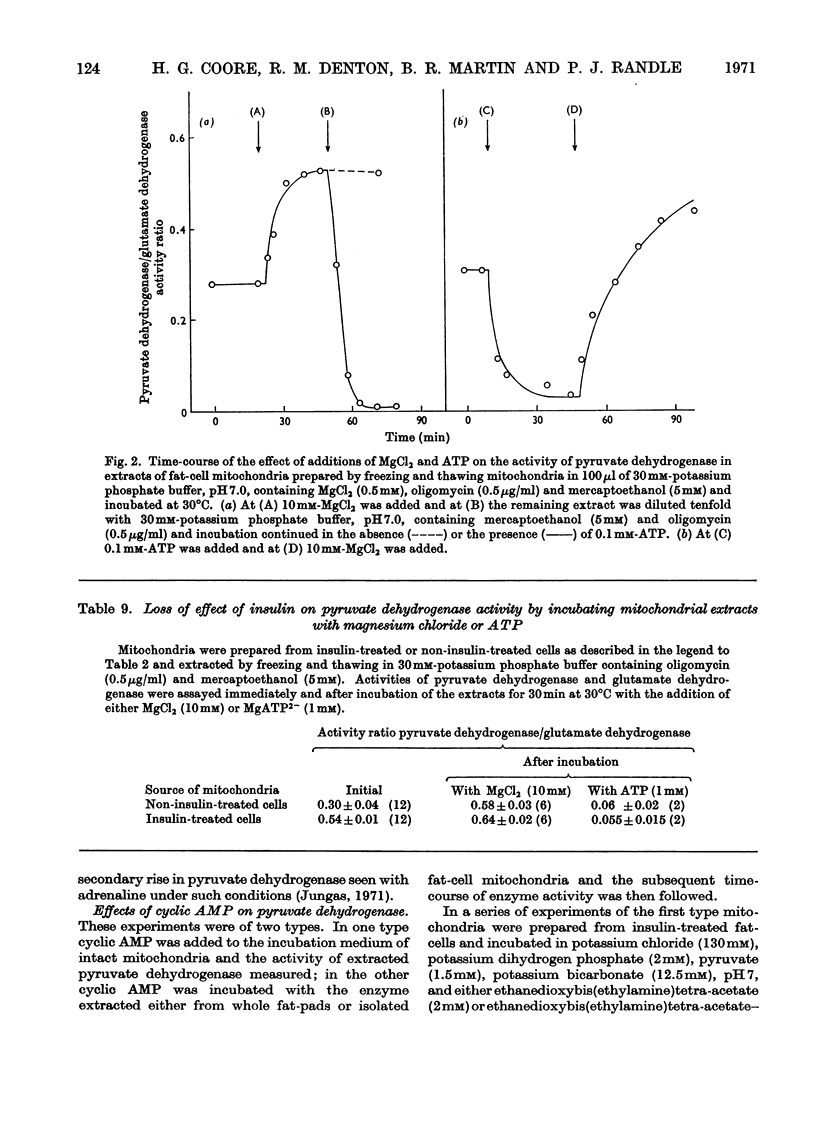
1. In epididymal adipose tissue synthesizing fatty acids from fructose in vitro, addition of insulin led to a moderate increase in fructose uptake, to a considerable increase in the flow of fructose carbon atoms to fatty acid, to a decrease in the steady-state concentration of lactate and pyruvate in the medium, and to net uptake of lactate and pyruvate from the medium. It is concluded that insulin accelerates a step in the span pyruvate→fatty acid. 2. Mitochondria prepared from fat-cells exposed to insulin put out more citrate than non-insulin-treated controls under conditions where the oxaloacetate moiety of citrate was formed from pyruvate by pyruvate carboxylase and under conditions where it was formed from malate. This suggested that insulin treatment of fat-cells led to persistent activation of pyruvate dehydrogenase. 3. Insulin treatment of epididymal fat-pads in vitro increased the activity of pyruvate dehydrogenase measured in extracts of the tissue even in the absence of added substrate; the activities of pyruvate carboxylase, citrate synthase, glutamate dehydrogenase, acetyl-CoA carboxylase, NADP–malate dehydrogenase and NAD–malate dehydrogenase were not changed by insulin. 4. The effect of insulin on pyruvate dehydrogenase activity was inhibited by adrenaline, adrenocorticotrophic hormone and dibutyryl cyclic AMP (6-N,2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate). The effect of insulin was not reproduced by prostaglandin E1, which like insulin may lower the tissue concentration of cyclic AMP (adenosine 3′:5′-cyclic monophosphate) and inhibit lipolysis. 5. Adipose tissue pyruvate dehydrogenase in extracts of mitochondria is almost totally inactivated by incubation with ATP and can then be reactivated by incubation with 10mm-Mg2+. In this respect its properties are similar to that of pyruvate dehydrogenase from heart and kidney where evidence has been given that inactivation and activation are catalysed by an ATP-dependent kinase and a Mg2+-dependent phosphatase. Evidence is given that insulin may act by increasing the proportion of active (dephosphorylated) pyruvate dehydrogenase. 6. Cyclic AMP could not be shown to influence the activity of pyruvate dehydrogenase in mitochondria under various conditions of incubation. 7. These results are discussed in relation to the control of fatty acid synthesis in adipose tissue and the role of cyclic AMP in mediating the effects of insulin on pyruvate dehydrogenase.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Effects of insulin and adrenaline on rat epididymal-fat-pad pyruvate dehydrogenase. Biochem J. 1971 Jul;123(4):38P–39P. doi: 10.1042/bj1230038pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Coore H. G., Martin B. R., Randle P. J. Insulin activates pyruvate dehydrogenase in rat epididymal adipose tissue. Nat New Biol. 1971 May 26;231(21):115–116. doi: 10.1038/newbio231115a0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Martin B. R., Coore H. G., Randle P. J. Properties of pyruvate dehydrogenase from fat-cell mitochondria and its hormonal control. Biochem J. 1971 Jul;123(4):39P–39P. doi: 10.1042/bj1230039pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Measurement of flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol and glycerol in rat heart and epididymal adipose tissue. Effects of insulin, adrenaline and alloxan-diabetes. Biochem J. 1967 Aug;104(2):423–434. doi: 10.1042/bj1040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Yorke R. E., Randle P. J. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J. 1966 Aug;100(2):407–419. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Halperin M. L. An additional role for insulin in the control of fatty acid synthesis independent of glucose transport. Can J Biochem. 1970 Nov;48(11):1228–1233. doi: 10.1139/o70-191. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Denton R. M. Regulation of glycolysis and L-glycerol 3-phosphate concentration in rat epididymal adipose tissue in vitro. Role of phosphofructokinase. Biochem J. 1969 Jun;113(1):207–214. doi: 10.1042/bj1130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L., Robinson B. H. The role of the cytoplasmic redox potential in the control of fatty acid synthesis from glucose, pyruvate and lactate in white adipose tissue. Biochem J. 1970 Jan;116(2):235–240. doi: 10.1042/bj1160235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp D., Challoner D. R., Williams R. H. Respiration in isolated fat cells and the effects of epinephrine. J Biol Chem. 1968 May 10;243(9):2321–2327. [PubMed] [Google Scholar]
- Huttunen J. K., Steinberg D., Mayer S. E. Protein kinase activation and phosphorylation of a purified hormone-sensitive lipase. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1350–1356. doi: 10.1016/0006-291x(70)90237-8. [DOI] [PubMed] [Google Scholar]
- JACOBSON K. B. Effect of substrate structure on activity of pigeon liver acetyl transferase. J Biol Chem. 1961 Feb;236:343–348. [PubMed] [Google Scholar]
- Jungas R. L. Effect of insulin on fatty acid ynthesis from pyruvate, lactage, or endogenous sources in adipose tissue: evidence for the hormonal regulation of pyruvate dehydrogenase. Endocrinology. 1970 Jun;86(6):1368–1375. doi: 10.1210/endo-86-6-1368. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Role of cyclic-3',5'-amp in the response of adipose tissue to insulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):757–763. doi: 10.1073/pnas.56.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loten E. G., Sneyd J. G. An effect of insulin on adipose-tissue adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1970 Nov;120(1):187–193. doi: 10.1042/bj1200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D. B., VAGELOS P. R. The mechanism of tricarboxylic acid cycle regulation of fatty acid synthesis. J Biol Chem. 1962 Jun;237:1787–1792. [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. Metabolism of pyruvate and malate by isolated fat-cell mitochondria. Biochem J. 1971 Nov;125(1):105–113. doi: 10.1042/bj1250105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZACK M. A. ACTIVATION OF AN EPINEPHRINE-SENSITIVE LIPOLYTIC ACTIVITY FROM ADIPOSE TISSUE BY ADENOSINE 3',5'-PHOSPHATE. J Biol Chem. 1964 Feb;239:392–395. [PubMed] [Google Scholar]
- ROBINSON B. H., WRIGHT P. H. Guinea-pig anti-insulin serum. J Physiol. 1961 Feb;155:302–310. doi: 10.1113/jphysiol.1961.sp006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J. 1970 Sep;119(2):221–242. doi: 10.1042/bj1190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E., Wittmann J., Wieland O. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):447–452. doi: 10.1515/bchm2.1971.352.1.447. [DOI] [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Wieland O., Siess E. Interconversion of phospho- and dephospho- forms of pig heart pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1970 Apr;65(4):947–954. doi: 10.1073/pnas.65.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]


