Abstract

Sulforaphane is a main bioactive component in several edible cruciferous vegetables. It acquires several benefits to health in addition to its considered antibacterial and antivirulence activities. Herein, we aimed at evaluating the antivirulence activity of sulforaphane against the worldwide clinically important enteric pathogen Salmonella enterica serovar Typhimurium. The influence of sulforaphane on bacterial adhesion, invasion, biofilm formation, and intracellular replication was assayed. Additionally, the effect of sulforaphane on the type III secretion system (TTSS) in S. enterica was quantified. The outcome of the combination with different antibiotics was assessed, and an in vivo protection assay was conducted to assess the influence on S. enterica pathogenesis. The results showed the significant antibiofilm activity of sulforaphane at subinhibitory effect in addition to its significant reduction in bacterial invasion and intracellular replication inside the host cells. The in vivo findings emphasized the decreased capacity of S. enterica to induce pathogenesis in the presence of sulforaphane. Our finding attributed these antivirulence activities to the interference of sulforaphane with TTSS-type II and the downregulation of its encoding genes. In a nutshell, the edible cruciferous vegetable bioactive sulforaphane is a safe adjunct therapy that can be administrated alongside traditional antibiotics for treating clinically significant enteric pathogens as S. enterica.
1. Introduction
Sulforaphane, a naturally occurring isothiocyanate found in cruciferous vegetables such as cabbage, broccoli, and kale, has garnered interest for its potential health benefits.1 These benefits include antioxidant and anti-inflammatory properties and the ability to stimulate detoxification enzymes.2,3 Additionally, sulforaphane has demonstrated noteworthy antibacterial effects against Gram-negative bacteria such as Pseudomonas aeruginosa,4Haemophilus influenzae,5 and enteric pathogens such as Escherichia coli,6Shigella sonnei,5 and Helicobacter pylori,7−9 as well as Gram-positive bacteria, including Streptococcus pyogenes and Staphylococcus aureus.10,11 Owing to the aforementioned health benefits of sulforaphane and its safety at higher concentrations, it is used as a food supplement.1 Interestingly, l-sulforaphane has garnered significant attention, as several studies, reviewed by Mazarakis et al.,12 have focused exclusively on the clinical evidence of the l-form activity. That inspired us to investigate the antibacterial activity of l-sulforaphane against one of the most clinically significant gut pathogens, Salmonella enterica.
S. enterica, belonging to the Enterobacteriaceae family, is a nonlactose fermenting, facultatively anaerobic, peritrichous motile, intracellular Gram-negative bacterium.13,14 Infections caused by S. enterica can range from localized gastroenteritis to severe systemic illness such as typhoid fever.14,15 Importantly, and due to easy transmission via contaminated food and water, S. enterica continues to pose substantial challenges to public health owing to its ability to cause widespread and severe epidemics.16 For instance, the observed increased incidence of salmonellosis in Ha’il, Saudi Arabia, where the cases were increasingly recorded in the time period 2013–2017 due to the contamination and improper preservation of food materials.17Salmonella employs complex type III secretion systems (TTSS) to translocate effector proteins into the cytoplasm of the host cells.18,19 Two separate TTSSs encoded in Salmonella pathogenicity islands, namely, SPI1 and SPI2 are present.20,21 In the early stages of the Salmonella invasion, SPI1-TTSS translocates effector proteins into the cytoplasm of the host cell to remodel the G-proteins easing the bacterial invasion.18,22 After the internalization of the Salmonella cells, the immune cells are contained in a specialized phagosome known as Salmonella-containing vacuoles (SCV). In SCV, immune cells produce diverse oxidizing agents and enzymes to eradicate the bacterial cells.18,23 However, SPI2-TTSS translocates a wide array of effectors that guarantee the survival of Salmonella and secure the delivery of nutrition.24 Importantly, TTSS interplays with other systems to control the bacterial adhesion, invasion, and establishment of serious infections in the host tissues.18,21,25
The number of new antibiotics developed and approved has declined steadily over the past few decades, leaving fewer options for combating resistant bacteria.26−29 Consequently, innovative solutions and alternative therapies are urgently needed to address this critical gap.28,30−33 The use of natural products to discover new antibacterials is a promising avenue for addressing antibiotic resistance.32−35 These compounds offer diverse and novel mechanisms of action that could lead to the development of effective new treatments.27,36,37 Interestingly, the antivirulence activity of sulforaphane at subinhibitory concentration against P. aeruginosa was approved. The antivirulence activity of sulforaphane was owed to its ability to antagonize the bacterial quorum sensing (QS) systems that control the expression of diverse virulence factors in P. aeruginosa.4 Furthermore, sulforaphane inactivated the H. pylori urease that resulted in diminishing the pathogenesis.8 In addition to the previously suggested mechanism of the sulforaphane’ s antivirulence activity, it inhibited the intracellular survival of H. pylori(7) and S. aureus.11 These data encourage us to further explore the antivirulence properties of sulforaphane against another clinically important pathogen S. enterica serovar Typhimurium (S. Typhimurium), evaluating its effect on TTSS and its influence on the Salmonella pathogenesis.
2. Materials and Methods
2.1. Bacteria, Media, and Chemicals
l-Sulforaphane was obtained from Cayman Chemical (Michigan). The antibacterial experiments were performed against S. enterica serovar Typhimurium NCTC 12023. The used microbiological media were obtained from Oxoid Ltd. (United Kingdom). Dimethyl sulfoxide (DMSO) was used to dissolve sulforaphane at a concentration of 0.5% v/v (2.8 mM). All other chemicals and solvents are of pharmaceutical grade.
2.2. Determination of Minimum Inhibitory Concentration (MIC)
MIC of sulforaphane against S. typhimurium was determined using the broth microdilution method according to the guidelines of the Clinical Laboratory and Standards Institute (CLSI, 2015) as previously detailed.35
2.3. Influence of Subinhibitory Concentrations on Bacterial Growth
To eliminate any influence of sulforaphane on S. typhimurium growth, viable bacterial counts were performed on S. typhimurium cultured in Luria–Bertani (LB) broth supplied or not with sulforaphane at sub-MIC.38
2.4. Adhesion and Biofilm Assay
Fresh cultures of S. typhimurium were grown in Tryptic Soy Broth (TSB) supplied or not with sulforaphane at sub-MIC. Bacterial suspensions, at a cell density of 2 × 106 CFU/mL, were then transferred to polystyrene microtiter plates and exposed to 0.001 μM N-hexanoyl-dl-homoserine lactone at 37 °C for either 2 or 24 h to assess bacterial adhesion or biofilm formation, respectively.20,39 Following the incubation periods, nonadherent bacterial cells were rinsed out, and the remaining stained cells were fixed with ethanol 95% at 60 °C for 25 min and then stained with 0.1% crystal violet for 15 min. Excess dye was removed, and the crystal violet was extracted with methanol before the optical density was measured at 590 nm.31,40
2.5. Internalization in the Host Cells
The invasion and intracellular replication assays were conducted to assess the ability of S. typhimurium to invade host cells and replicate within them, respectively.20,22,41 For the invasion assay, host cells (HeLa cells) were seeded in 24 well plates at a density of 5 × 105 cells/well and infected with S. typhimurium treated or not treated with sulforaphane at a sub-MIC at a multiplicity of infection (MOI) of 1. After a specified incubation period (30 min), noninternalized bacteria were washed out, and the remaining nonadhered extracellular Salmonella were killed with gentamicin (100 μg/mL) for 1 h. The invaded bacteria were then quantified by lysing host cells with Triton X-100 (0.1%) for 20 min at 20 °C, and the cell lysates were serially diluted and counted.
In the intracellular replication assay, infected host cells (raw macrophages (RAW264.7)) were incubated for specific durations to allow bacterial replication. At predetermined time points (2 and 16 h postinfection), the macrophages were lysed with 0.1% Triton X-100, and the intracellular bacteria were quantified by plating lysates on appropriate agar plates. The ratio of intracellular bacteria at later time points compared with the initial inoculum was calculated to determine the fold increase in intracellular replication.
2.6. Assay of the SPI2 Effector Translocation
The translocation of the SPI2 effector SseJ was used to assess the effect of sulforaphane at sub-MIC on the effectiveness of SPI2-T3SS in S. typhimurium survival and induction of infection in host cells. The plasmid pWsk29 PsseJsseJ::hSurvivin that encodes the hemagglutinin (HA)-tagged SPI2 effector protein SseJ was constructed and transformed to S. typhimurium as detailed.20,22 The obtained S. typhimurium carrying the plasmid was cultivated with or without sulforaphane at subinhibitory concentration. These bacterial suspensions were then used to infect macrophages or HeLa cells in the presence of 0.001 μM N-hexanoyl-dl-homoserine lactone at an MOI of 100.20,22,41 The infected cells were immune-stained to assess the translocated effector proteins after 16 h, using antibodies against Salmonella LPS (rabbit anti-Salmonella O1,4,5, Difco, BD) and the HA epitope tag (Roche, Basel, Switzerland). Secondary antibodies were used as follows: antirabbit antibodies tagged with GFP (green fluorescent protein) to stain Salmonella cells (Abcam), Cyanine5 (Cy5) dye to stain the translocated SseJ::HA (Invitrogen, MA), and diamidino-2-phenylindole (DAPI) dye as a counter stain for macrophages (Thermo Fisher Scientific).19,20,22 A Leica laser scanning confocal microscope was used to capture the images of infected cells and translocated SseJ::HA. The fluorescence intensities of SseJ::HA were quantified using the J-image program.
2.7. Expression of the TTSS-SPI2 Genes
SPI2-inducing minimal medium (PCN-P, pH 5.8) was used to induce the SPI2 effectors’ expression in the presence of sulforaphane at sub-MIC.24 RNA was isolated from the bacterial cultures using RNAeasy Mini Kit (Qiagen, Germany), kept and stored at −80 °C.42,43 cDNA was acquired through the utilization of the high-capacity cDNA reverse transcriptase kit (Applied Biosystem), and subsequently, it was amplified using the Syber Green I PCR Master Kit (Fermentas) according to the provided protocol.42,44 Relative expression of tested genes was calculated using the 2–ΔΔCT method and normalized to housekeeping gene gyrB, the used primers were previously listed.41
2.8. Combination with Antibiotics
The outcome of sulforaphane at sub-MIC in combination with antibiotics, at their MICs, was assessed using the checkerboard method.38,45 The antibiotics chosen for evaluation represent different classes and include chloramphenicol, cefotaxime, ciprofloxacin, gentamicin, amoxycillin-clavulanic acid, and tetracycline. The effectiveness of the combinations was evaluated using the fractional inhibitory concentration (FIC) index, determined by dividing MIC of the antibiotic in combination with MIC of the antibiotic solely. Optical densities were recorded at 600 nm following a 24 h incubation. FIC index values falling between 0.5 and 4 signified an indifferent effect; those exceeding 4 indicated antagonism, while values below 0.5 suggested synergy.
2.9. Protection of Mice
To investigate the in vivo suppressive effect of sulforaphane at sub-MIC on S. typhimurium pathogenesis, albino mice (3 weeks old) received intraperitoneal injections (I.P) of bacteria that had been treated with sulforaphane at sub-MIC, dissolved in DMSO (0.5% v/v).36,46 The experiment included four groups with 5 mice in each. Group I received S. typhimurium (1 × 106 CFU/mL) treated with sulforaphane at sub-MIC. Group II served as positive control and was injected with S. typhimurium. Groups III and IV were left uninfected or injected with DMSO (0.5%, v/v) and served as negative controls. Following 1 week of monitoring, the mice were euthanized, and their liver and kidney tissues were dissected and homogenized. Bacterial loads were precisely determined through serial dilution in PBS and plating onto Salmonella Shigella agar (SSA) plates. The bacterial loads within the organs were evaluated in terms of the number of colony-forming units (CFU)/ g tissue.
2.10. Statistical Evaluations
The reported data represent mean values ± standard deviation (SD) in comparison to untreated controls for all assays, except for the evaluation of the effect of sulforaphane on the downregulation of involved genes, where mean values ± standard error (SE) were calculated. Unless specified otherwise, significance was assessed using the student’s t test (p < 0.05).
3. Results
3.1. Effect of Sulforaphane at Sub-MIC on the Bacterial Growth
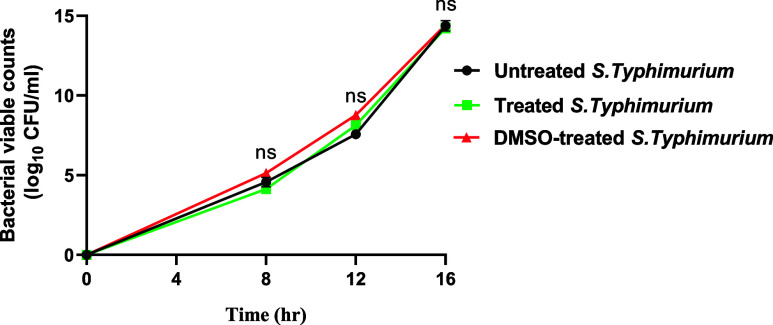
Sulforaphane prevented the S. typhimurium growth at 8 μg/mL. Accordingly, to investigate the antivirulence activity of sulforaphane while excluding its effect on bacterial growth, all of the next experiments were conducted using sulforaphane at sub-MIC (1/4 MIC = 2 μg/mL). Additionally, viable counts of S. typhimurium cultured in the media provided with sulforaphane at sub-MIC (2 μg/mL) were conducted and compared with those of untreated control bacteria (Figure 1). A one-way ANOVA test was used to calculate the statistical significance.
Figure 1.
Impact of sulforaphane at sub-MIC on bacterial growth. The viable counts of S. typhimurium cultured in media supplied or not with sulforaphane were performed. There were no significant differences between cultures treated with or not with sulforaphane. ns: nonsignificant, p > 0.05.
3.2. Antiadhesive and Antibiofilm Activities of Sulforaphane at Sub-MIC
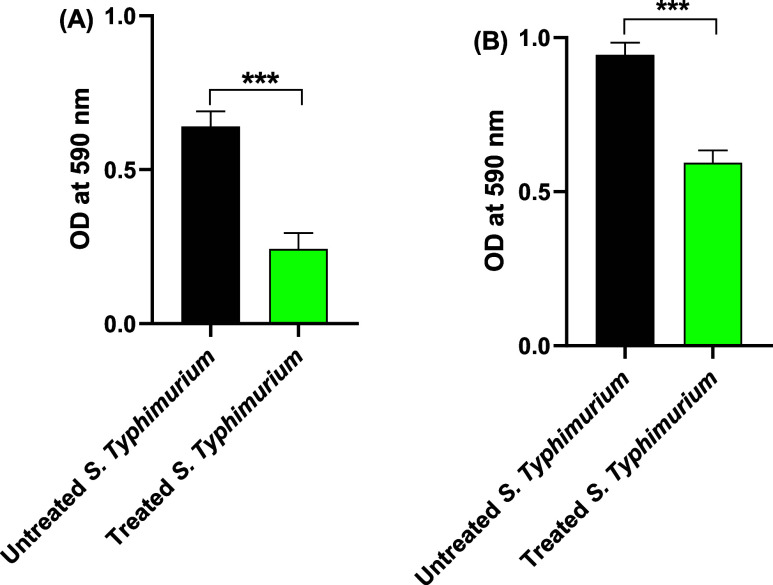
To evaluate the efficacy of sulforaphane at a sub-MIC level (2 μg/mL) in disrupting S. typhimurium adhesion and biofilm formation, spectrophotometric measurement of crystal violet intensity on adhered cells was conducted after 1 and 24 h (Figure 2). Sulforaphane at sub-MIC levels significantly attenuated bacterial adhesion and biofilm formation. Specifically, it decreased bacterial adhesion by 62% and biofilm formation by 37% at subinhibitory concentrations.
Figure 2.
Sulforaphane at sub-MIC significantly lessened the (A) adhesion and (B) biofilm formation of S. typhimurium to abiotic surfaces. ***p < 0.001.
3.3. Effect of Sulforaphane at Sub-MIC on the Salmonella Internalization
Salmonella utilizes TTSS for initiating infection and survival intracellularly within the phagosomes of host immune cells. To investigate the potential of sulforaphane to disrupt S. typhimurium invasion and intracellular replication, viable bacteria were quantified in HeLa cells and macrophages, respectively, in the presence and absence of sulforaphane at sub-MIC levels (2 μg/mL) (Figure 3). The bacterial counts of invading bacterial cells in HeLa cells and the intracellularly replicated bacteria were significantly reduced in the presence of sulforaphane at sub-MIC. Sulforaphane at sub-MIC reduced the bacterial invasion by 46% and intracellular replication by 55%.
Figure 3.
Sulforaphane at sub-MIC significantly diminished the (A) invasion and (B) intracellular replication of S. typhimurium into HeLa and raw macrophages, respectively. ***p < 0.001.
3.4. Interference of Sulforaphane at Sub-MIC with TTSS-SPI2
Bearing in mind that TTSS plays a crucial role in the survival of S. typhimurium in host cells, the functionality of TTSS in the presence of sulforaphane at sub-MIC (2 μg/mL) was assessed by quantifying the translocation of the SPI2 effectors into the cytoplasm of the host cells. The intensity of the red fluorescence resulting from cy5-stained translocated SPI2 effector (SseJ::HA) was quantified using HeLa cells and macrophages (Figure 4).
Figure 4.
Sulforaphane significantly hindered the translocation of S. typhimurium SPI2 effector. The red fluorescence intensity (cy5) of the translocated SseJ::HA was quantified in the (A) HeLa and (B) raw macrophages infected with S. typhimurium treated with sulforaphane at sub-MIC. The Bacterial cells were counterstained with GFP-tagged secondary antibodies appearing green, while the macrophages were stained with DAPI. The intensity was measured at least in 30 cells infected with similar numbers of bacterial cells. Sulforaphane significantly diminished the translocation of the SPI2 effector (***p < 0.001).
3.5. Downregulation Effect of Sulforaphane on the TTSS-SPI2 Genes at Sub-MIC
Sulforaphane at sub-MIC significantly downregulated the expression of several SPI2 effectors, and more downregulation was observed in genes ssrB, sseJ, sifA, and sifB (3–5-fold). While the downregulation effect was 2-fold in genes ssaE, ssaJ, and sseF, the expression of steC and ssaV genes did not exceed one-fold. On the other hand, sulforaphane has no influence on the expression of sseL, sscA, and pipB genes (Figure 5).
Figure 5.
Sulforaphane at sub-MIC downregulated the expression of some SPI2 genes. The expression levels were normalized to the gyrB gene. ns: nonsignificant, *p < 0.05, **p < 0.01, and ***p < 0.001.
3.6. Outcome of the Sulforaphane Combination with Antibiotics
The MIC values for combinations of sulforaphane at subminimal inhibitory concentration (2 μg/mL) with the antibiotics under examination (ciprofloxacin, streptomycin, ceftazidime, amoxycillin/clavulanic acid, and chloramphenicol) were determined, and the FIC index was computed to assess the effects of these combinations (Table 1). As depicted in Table 1, sulforaphane reduced MICs of the antibiotics tested, indicating synergistic effects. The calculated FIC values (in all combinations) were consistently less than 0.5, clearly indicating the synergistic effect of combining sulforaphane with other antibiotics.
Table 1. Susceptibility to Antibiotics in the Presence of Sulforaphane at Sub-MIC.
| antibiotic | MICs (μg/mL) | MICantibiotic+sulf (μg/mL) | FIC |
|---|---|---|---|
| ciprofloxacin | 4 | 0.5 | 0.25 |
| ceftazidime | 56 | 32 | 0.5 |
| amoxycillin/clavulanic acid | 256 | 128 | 0.5 |
| streptomycin | 8 | 2 | 0.25 |
| chloramphenicol | 256 | 32 | 0.125 |
MICs of antibiotics such as ciprofloxacin, streptomycin, ceftazidime, amoxycillin/clavulanic acid, and chloramphenicol was detected against S. typhimurium. MIC of combinations of sulforaphane at subminimal inhibitory concentration (2 μg/mL) with the antibiotics was detected. The FIC index was calculated to evaluate the outcome of these combinations. Sulforaphane lessened MICs of the tested antibiotics showing synergistic outcomes.
3.7. Protection of Sulforaphane to the Mice against Salmonella Infection
Sulforaphane at sub-MIC conferred 50% protection to the mice infected with S. typhimurium. There were four deaths observed among the positive control group injected with S. typhimurium, while this number was reduced to two in the group injected with S. typhimurium pretreated with sulforaphane. The survival curve (Figure 6A) clearly revealed a significant reduction in the S. typhimurium pathogenesis and its ability to induce infection in the presence of sulforaphane (p = 0.0114) (Figure 6A). Of note, no deaths were observed in the negative control groups. Most importantly, the counting of bacterial loads in the isolated kidney and live tissues revealed significant reductions in the bacterial counts upon treatment with sulforaphane (Figure 6B,C).
Figure 6.

Sulforaphane significantly protected mice against S. typhimurium. (A) Survival curve for S. typhimurium-treated mice (n = 5) in the presence or absence of sulforaphane. The bacterial load in the isolated (B) liver and (C) kidney tissues. *p < 0.05, **p < 0.01, and ***p < 0.001.
4. Discussion
S. enterica is a common cause of foodborne illness worldwide that ranges from mild gastroenteritis to severe enteric fever.13,47 During the past decade, there was a notable increase in the incidence of salmonellosis in Ha’il, Saudi Arabia, probably due to the contamination and improper preservation of food materials.17 Sulforaphane is a prominent component in the cruciferous vegetables and acquires considerable antimicrobial activities against several enteric pathogens.3,4 Furthermore, the safety of sulforaphane has been approved at high concentrations and can be provided as a food supplement.8,48 Sulforaphane showed significant antivirulence activities employing several mechanisms targeting urease in H. pylori(7,8) and QS systems in P. aeruginosa,33,49 importantly, it inhibits the intracellular survival of S. aureus(11) and H. pylori.7Salmonella is an intracellular bacterium that can survive inside the phagosome of host immune cells recruiting specialized injectosome “TTSS” to cope the adverse conditions.22,50 Bearing in mind these findings, it was hypothesized that sulforaphane could acquire activity against S. typhimurium, placing particular emphasis on examining its impact on TTSS as well as bacterial internalization inside host cells.
Sulforaphane inhibited the S. typhimurium growth at a considerable low concentration; however, its antivirulence activities were assayed using 1/4 MIC (2 μg/mL) to exclude any influence on growth. Bacteria employ diverse systems to induce pathogenesis, mainly QS systems, which play diverse roles in orchestrating bacterial virulence including the production of virulence factors and biofilm formation in both Gram-negative and Gram-positive bacteria.51−53 In Salmonella, QS significantly contributes to its pathogenicity playing a crucial role in regulating virulence, biofilm formation, and interaction with the host environment.54,55 In our previous study, sulforaphane at sub-MIC diminished the production of virulence factors and biofilm formation in P. aeruginosa, which in turn decreased the bacteria’s capability to establish infection in the mice. These antivirulence activities and antibiofilm were attributed to the sulforaphane’s interference with the QS systems and downregulation of their encoding genes.4 In compliance with these results, sulforaphane at sub-MIC diminished S. typhimurium adhesion and biofilm formation. That could be attributed to the anti-QS activities of sulforaphane.4
The intracellular accumulation of sulforaphane to high levels resulted in the eradication of the intracellular resistant H. pylori.7 Additionally, sulforaphane showed considered ability to diminish the intracellular survival of S. aureus via inhibition of mitogen-activated protein kinase (MAPK) (p38) and c-Jun N-terminal kinase (JNK) MAPK signaling pathways in macrophages.11 Interestingly, sulforaphane significantly decreased the S. typhimurium invasion and intracellular replication, which is in agreement with the others’ findings mentioned above. However, in an intracellular pathogen such as Salmonella, the mechanism of sulforaphane’s antivirulence is quite different as it depends on its interference with TTSS. TTSS is an injectosome encoded mainly by two clusters of genes localized in SPI1 and SPI2 to produce two types of TTSS.13,18 Although the first type of TTSS is involved in the early stages of Salmonella invasion, the functions of the second type are more important and essential to the bacterial survival inside the host cells.18,56 The TTSS machinery is working via translocation of effector proteins to remodel the host cells and ease the Salmonella invasion, intracellular replication, and then establish an infection in host cells.22 In this direction, the influence of sulforaphane at the sub-MIC on the effector translocation was assayed. The translocation of HA-tagged SseJ (SPI2 effector protein) was quantified in HeLA cells and macrophages; sulforaphane significantly diminished the translocation of the SPI2 effector that indicates its interference with TTSS and explains the significant reduction in the Salmonella intracellular replication.
The second type of TTSS is encoded by more than 30 genes that encode the (i) structural apparatus (Ssa) of TTSS, (ii) effectors (Sse) that are translocated in the host cytoplasm easing the bacterial cope inside phagosomes, (iii) chaperones (Ssc) that are required to keep the functions of effectors, and (iv) regulatory secretion system SsrAB that is located in a separate operon.13,56−58 Sulforaphane at sub-MIC significantly downregulated the regulatory gene ssrB as well as apparatus encoding genes ssaE and ssaJ; however, there was no significant effect on the expression of ssaV. Sulforaphane downregulated the expression of effectors encoding genes sseF, steC, and sseJ, but there was no effect on the expression of the sseL gene. The genes sseF and sseJ encode essential effectors for maintaining the integrity of SCV,56,57 while SteC manipulates the host cell actin cytoskeleton and modulates signaling pathways.59Salmonella spreads a dynamic network of filaments known as Salmonella-induced filaments (SIFs) that are crucial for the bacterial to thrive intracellularly.60 Our results showed that sulforaphane significantly decreased the expression of SIF formation and microtubule bundling encoding genes sifA and sifB.57 On the other hand, sulforaphane has no effect on gene encodes PipB which plays a role in the SCV trafficking along microtubules securing the Salmonella survival.23,61 In addition, sulforaphane has no significant effect on the expression of chaperone encoding gene sscA. These findings attest to the downregulating influence of sulforaphane at sub-MIC on SPI2 genes that could explain the lessened SPI2 effector translocation and in turn the reduction in the intracellular replication.
To ensure the above in vitro results, a mice protection assay was conducted to evaluate the capacity of sulforaphane to diminish the S. typhimurium pathogenesis. Intriguingly, sulforaphane at sub-MIC significantly saved mice against S. typhimurium and also significantly decreased the bacterial colonization in the isolated liver and kidney tissues. That is in great alignment with the interference of sulforaphane with TTSS and a decrease in the invasion and intracellular replication abilities. One of the significant challenges in treating Salmonella infections is the development of antibiotic resistance. Sulforaphane may offer a complementary approach to combat antibiotic-resistant strains by targeting different mechanisms than conventional antibiotics. In this study, sulforaphane showed significant antivirulence activities and exerted a synergistic effect with various antibiotics even at low concentrations (at sub-MIC levels). The current study proposes utilizing l-sulforaphane as an antivirulence agent at 2 μg/mL, which is a concentration readily obtainable from just a few grams of cruciferous vegetables, such as broccoli.1 Additionally, sulforaphane is safe at high concentrations and can be easily delivered with diet in the form of edible cruciferous vegetables.48,62 This could emphasize its possible administration as an adjunct to traditional antibiotics to overcome the resistant enteric bacterial infections such as H. pylori(7,8) and S. typhimurium.
5. Conclusions
Sulforaphane is a predominant bioactive component in edible cruciferous plants. Besides, the sulforaphane anti-inflammatory and antioxidant known activities acquire significant antibacterial effects in particular against intracellular enteric pathogens. Our findings emphasized the sulforaphane interference with TTSS, which plays a key role in the enteric pathogen S. enterica invasion and intracellular replication. Furthermore, sulforaphane possesses antibiofilm activity that is in compliance with its previously proved anti-QS activity. In vivo findings attested to the in vitro results, where sulforaphane protected mice against S. typhimurium and decreased the bacterial colonization. Sulforaphane showed synergistic outcomes when combined with different antibiotics. To sum up, sulforaphane, which is abundantly present in edible cruciferous vegetables and can be directly delivered to the stomach, can serve as a safe adjuvant to antibiotics to treat clinically important enteric pathogens. However, further pharmacological and pharmaceutical investigations are necessary before considering the potential clinical use of sulforaphane.
Acknowledgments
This research was funded by the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number (RCP-24 067).
Author Contributions
S.M.D.R.: Methodology. A.S.A.L., A.M., and E.-S.K.: Investigation, Visualization, Funding acquisition. A.A.H.R. and M.M.B.: Methodology, Validation, Investigation. W.A.H.H.: Conceptualization, Writing—original draft, Supervision, Administration.
The authors declare no competing financial interest.
Notes
Ethical Approval The Faculty of Pharmacy, Port Said University Ethical Committee, allowed in vivo mouse tests in this study. The experiments were conducted in agreement with the ARRIVE guidelines and the UK Animals Act of 1986 (Accession no. PSU.PHR.19).
References
- Yagishita Y.; Fahey J. W.; Dinkova-Kostova A. T.; Kensler T. W. Broccoli or sulforaphane: is it the source or dose that matters?. Molecules 2019, 24 (19), 3593 10.3390/molecules24193593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimognari C.; Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res., Rev. Mutat. Res. 2007, 635 (2–3), 90–104. 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]; Guerrero-Beltrán C. E.; Calderón-Oliver M.; Pedraza-Chaverri J.; Chirino Y. I. Protective effect of sulforaphane against oxidative stress: recent advances. Exp. Toxicol. Pathol. 2012, 64 (5), 503–508. 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Briones-Herrera A.; Eugenio-Pérez D.; Reyes-Ocampo J. G.; Rivera-Mancía S.; Pedraza-Chaverri J. New highlights on the health-improving effects of sulforaphane. Food Funct. 2018, 9 (5), 2589–2606. 10.1039/C8FO00018B. [DOI] [PubMed] [Google Scholar]
- Bendary M. M.; Ali M. A.; Halim A. S. A.; Boufahja F.; Chaudhary A. A.; Elkelish A.; Soliman R. H.; Hegazy W. A. Investigating Sulforaphane’s anti-virulence and anti-quorum sensing properties against Pseudomonas aeruginosa. Front. Pharmacol. 2024, 15, 1406653 10.3389/fphar.2024.1406653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis N.; Higgins R. A.; Anderson J.; Toh Z. Q.; Luwor R. B.; Snibson K. J.; Karagiannis T. C.; Do L. A. H.; Licciardi P. V. The effects of the dietary compound L-sulforaphane against respiratory pathogens. Int. J. Antimicrob. Agents 2021, 58 (6), 106460 10.1016/j.ijantimicag.2021.106460. [DOI] [PubMed] [Google Scholar]
- Nowicki D.; Maciąg-Dorszyńska M.; Bogucka K.; Szalewska-Pałasz A.; Herman-Antosiewicz A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9 (1), 13677 10.1038/s41598-019-50216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. W.; Haristoy X.; Dolan P. M.; Kensler T. W.; Scholtus I.; Stephenson K. K.; Talalay P.; Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo [a] pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. U.S.A. 2002, 99 (11), 7610–7615. 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. W.; Stephenson K. K.; Wade K. L.; Talalay P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435 (1), 1–7. 10.1016/j.bbrc.2013.03.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haristoy X.; Angioi-Duprez K.; Duprez A.; Lozniewski A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47 (12), 3982–3984. 10.1128/AAC.47.12.3982-3984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M.-O.; Kim M.-B.; Lim S.-B. Relationship between chemical structure and antimicrobial activities of isothiocyanates from cruciferous vegetables against oral pathogens. J. Microbiol. Biotechnol. 2016, 26 (12), 2036–2042. 10.4014/jmb.1606.06008. [DOI] [PubMed] [Google Scholar]
- Deramaudt T. B.; Ali M.; Vinit S.; Bonay M. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation. Int. J. Mol. Med. 2020, 45 (6), 1927–1941. 10.3892/ijmm.2020.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis N.; Snibson K.; Licciardi P. V.; Karagiannis T. C. The potential use of l-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39 (3), 664–675. 10.1016/j.clnu.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Hegazy W. A. H.; Hensel M. Salmonella enterica as a vaccine carrier. Future Microbiol. 2012, 7 (1), 111–127. 10.2217/fmb.11.144. [DOI] [PubMed] [Google Scholar]
- Wain J.; Hendriksen R. S.; Mikoleit M. L.; Keddy K. H.; Ochiai R. L. Typhoid fever. Lancet 2015, 385 (9973), 1136–1145. 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle G. C.; Walker C. L.; Black R. E. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J. Global Health 2012, 2 (1), 010401 10.7189/jogh.01.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich C.; Hartman H.; Feasey N.; Chattaway M. A.; Dekker D.; Al-Emran H. M.; Larkin L.; McCormick J.; Sarpong N.; Le Hello S.; et al. Emergence of phylogenetically diverse and fluoroquinolone resistant Salmonella Enteritidis as a cause of invasive nontyphoidal Salmonella disease in Ghana. PLoS Neglected Trop. Dis. 2019, 13 (6), e0007485 10.1371/journal.pntd.0007485. [DOI] [PMC free article] [PubMed] [Google Scholar]; Crump J. A.; Sjolund-Karlsson M.; Gordon M. A.; Parry C. M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28 (4), 901–937. 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsayeqh A. F. Salmonellosis in Saudi Arabia; an underestimated disease?. Alexandria J. Vet. Sci. 2020, 67 (1), 30–38. 10.5455/ajvs.136155. [DOI] [Google Scholar]; Abouammoh N.; Training F. E.. Food Borne Disease Outbreak in Abqaiq (Security Facility campus).
- Cornelis G. R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4 (11), 811–825. 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Xu X.; Hegazy W. A.; Guo L.; Gao X.; Courtney A. N.; Kurbanov S.; Liu D.; Tian G.; Manuel E. R.; Diamond D. J.; et al. Effective cancer vaccine platform based on attenuated salmonella and a type III secretion system. Cancer Res. 2014, 74 (21), 6260–6270. 10.1158/0008-5472.CAN-14-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askoura M.; Almalki A. J.; Lila A. S. A.; Almansour K.; Alshammari F.; Khafagy E.-S.; Ibrahim T. S.; Hegazy W. A. H. Alteration of Salmonella enterica Virulence and Host Pathogenesis through Targeting sdiA by Using the CRISPR-Cas9 System. Microorganisms 2021, 9 (12), 2564 10.3390/microorganisms9122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfaky M. A.; Thabit A. K.; Eljaaly K.; Zawawi A.; Abdelkhalek A. S.; Almalki A. J.; Ibrahim T. S.; Hegazy W. A. H. Controlling of Bacterial Virulence: Evaluation of Anti-Virulence Activities of Prazosin against Salmonella enterica. Antibiotics 2022, 11 (11), 1585 10.3390/antibiotics11111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy W. A. H.; Xu X.; Metelitsa L.; Hensel M. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect. Immun. 2012, 80 (3), 1193–1202. 10.1128/IAI.06056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L. A.; Celli J.; Hardt W. D.; Vallance B. A.; Yip C.; Finlay B. B. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol. Microbiol. 2002, 43 (5), 1089–1103. 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- Deiwick J.; Nikolaus T.; Erdogan S.; Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 1999, 31 (6), 1759–1773. 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- Thabit A. K.; Eljaaly K.; Zawawi A.; Ibrahim T. S.; Eissa A. G.; Elbaramawi S. S.; Hegazy W. A. H.; Elfaky M. A. Silencing of Salmonella typhimurium Pathogenesis: Atenolol Acquires Efficient Anti-Virulence Activities. Microorganisms 2022, 10 (10), 1976 10.3390/microorganisms10101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M. British Society for Antimicrobial Chemotherapy Working Party on The Urgent Need: Regenerating Antibacterial Drug, D.; Development. Discovery research: the scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66 (9), 1941–1944. 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]; Mohr K. I. History of Antibiotics Research. Curr. Top. Microbiol. Immunol. 2016, 398, 237–272. 10.1007/82_2016_499. [DOI] [PubMed] [Google Scholar]
- Rajab A. A. H.; Hegazy W. A. What’s old is new again: Insights into diabetic foot microbiome. World J. Diabetes 2023, 14 (6), 680 10.4239/wjd.v14.i6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Lila A. S.; Alharby T. N.; Alanazi J.; Alanazi M.; Abdallah M. H.; Rizvi S. M. D.; Moin A.; Khafagy E.-S.; Tabrez S.; Al Balushi A. A.; Hegazy W. A. H. Clinical resistant strains of Enterococci and their correlation to reduced susceptibility to biocides: phenotypic and genotypic analysis of macrolides, lincosamides, and streptogramins. Antibiotics 2023, 12 (3), 461 10.3390/antibiotics12030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha K. A.; Abo-Dya N. E.; Ibrahim T. S.; Abdel-Aal E. H.; Hegazy W. A. Benzotriazole-Mediated Synthesis and Antibacterial Activity of Novel N-Acylcephalexins. Sci. Pharm. 2016, 84 (3), 484–496. 10.3390/scipharm84030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi H. F.; Alotaibi H.; Darwish K. M.; Khafagy E.-S.; Abu Lila A. S.; Ali M. A.; Hegazy W. A.; Alshawwa S. Z. The Anti-Virulence Activities of the Antihypertensive Drug Propranolol in Light of Its Anti-Quorum Sensing Effects against Pseudomonas aeruginosa and Serratia marcescens. Biomedicines 2023, 11 (12), 3161 10.3390/biomedicines11123161. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abbas H. A.; Hegazy W. A. H. Targeting the virulence factors of Serratia marcescens by ambroxol. Roum. Arch. Microbiol. Immunol. 2017, 76 (2), 27–32. [Google Scholar]
- Elfaky M. A.; Elbaramawi S. S.; Eissa A. G.; Ibrahim T. S.; Khafagy E.-S.; Ali M. A.; Hegazy W. A. Drug repositioning: Doxazosin attenuates the virulence factors and biofilm formation in Gram-negative bacteria. Appl. Microbiol. Biotechnol. 2023, 107 (11), 3763–3778. 10.1007/s00253-023-12522-3. [DOI] [PubMed] [Google Scholar]
- Khayat M. T.; Ibrahim T. S.; Khayyat A. N.; Alharbi M.; Shaldam M. A.; Mohammad K. A.; Khafagy E.-S.; El-damasy D. A.; Hegazy W. A. H.; Abbas H. A. Sodium Citrate Alleviates Virulence in Pseudomonas aeruginosa. Microorganisms 2022, 10 (5), 1046 10.3390/microorganisms10051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rabia M. W.; Asfour H. Z.; Alhakamy N. A.; Abdulaal W. H.; Ibrahim T. S.; Abbas H. A.; Salem I. M.; Hegazy W. A.; Nazeih S. I. Thymoquinone is a natural antibiofilm and pathogenicity attenuating agent in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2024, 14, 1382289 10.3389/fcimb.2024.1382289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat M. T.; Elbaramawi S. S.; Nazeih S. I.; Safo M. K.; Khafagy E.-S.; Ali M. A.; Abbas H. A.; Hegazy W. A.; Seleem N. M. Diminishing the Pathogenesis of the Food-Borne Pathogen Serratia marcescens by Low Doses of Sodium Citrate. Biology 2023, 12 (4), 504 10.3390/biology12040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr-Eldin S. M.; Aldawsari H. M.; Ahmed O. A.; Kotta S.; Abualsunun W.; Eshmawi B. A.; Khafagy E.-S.; Elbaramawi S. S.; Abbas H. A.; Hegazy W. A.; Seleem N. M. Utilization of zein nano-based system for promoting antibiofilm and anti-virulence activities of curcumin against Pseudomonas aeruginosa. Nanotechnol. Rev. 2024, 13 (1), 20230212 10.1515/ntrev-2023-0212. [DOI] [Google Scholar]
- Khayat M. T.; Abbas H. A.; Ibrahim T. S.; Elbaramawi S. S.; Khayyat A. N.; Alharbi M.; Hegazy W. A.; Yehia F. A.-z. A. Synergistic Benefits: Exploring the Anti-Virulence Effects of Metformin/Vildagliptin Antidiabetic Combination against Pseudomonas aeruginosa via Controlling Quorum Sensing Systems. Biomedicines 2023, 11 (5), 1442 10.3390/biomedicines11051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeih S. I.; Ali M. A.; Halim A. S. A.; Al-Lawati H.; Abbas H. A.; Al-Zharani M.; Boufahja F.; Alghamdi M. A.; Hegazy W. A.; Seleem N. M. Relocating Glyceryl Trinitrate as an Anti-Virulence Agent against Pseudomonas aeruginosa and Serratia marcescens: Insights from Molecular and In Vivo Investigations. Microorganisms 2023, 11 (10), 2420 10.3390/microorganisms11102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshak A. E.; Okairy H. M.; Elfaky M. A.; Abdallah H. M.; Mohamed G. A.; Ibrahim S. R.; Alzain A. A.; Abulfaraj M.; Hegazy W. A.; Nazeih S. I. Antimicrobial and anti-virulence activities of 4-shogaol from grains of paradise against gram-negative bacteria: Integration of experimental and computational methods. J. Ethnopharmacol. 2024, 323, 117611 10.1016/j.jep.2023.117611. [DOI] [PubMed] [Google Scholar]
- Stepanović S.; Vukovic D.; Dakic I.; Savic B.; Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40 (2), 175–179. 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Vesterlund S.; Paltta J.; Karp M.; Ouwehand A. C. Measurement of bacterial adhesion-in vitro evaluation of different methods. J. Microbiol. Methods 2005, 60 (2), 225–233. 10.1016/j.mimet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Askoura M.; Hegazy W. A. H. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog. Dis. 2020, 78 (1), ftaa011 10.1093/femspd/ftaa011. [DOI] [PubMed] [Google Scholar]
- Wael A. H. H.; Mohamed A. H. Hepatitis C virus pathogenesis: Serum IL-33 level indicates liver damage. Afr. J. Microbiol. Res. 2015, 9 (20), 1386–1393. 10.5897/AJMR2015.7496. [DOI] [Google Scholar]
- Koshak A. E.; Elfaky M. A.; Abdallah H. M.; Albadawi D. A. I.; Mohamed G. A.; Ibrahim S. R. M.; Alzain A. A.; Khafagy E. S.; Rajab A. A. H.; Hegazy W. A. H. Arctigenin from Burdock Root Exhibits Potent Antibacterial and Anti-Virulence Properties against Pseudomonas aeruginosa. J. Microbiol. Biotechnol. 2024, 34 (8), 1642–1652. 10.4014/jmb.2403.03003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askoura M.; Abbas H. A.; Al Sadoun H.; Abdulaal W. H.; Abu Lila A. S.; Almansour K.; Alshammari F.; Khafagy E.-S.; Ibrahim T. S.; Hegazy W. A. H. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens 2022, 11 (1), 57 10.3390/pathogens11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfaky M. A.; Okairy H. M.; Abdallah H. M.; Koshak A. E.; Mohamed G. A.; Ibrahim S. R.; Alzain A. A.; Hegazy W. A.; Khafagy E.-S.; Seleem N. M. Assessing the antibacterial potential of 6-gingerol: Combined experimental and computational approaches. Saudi Pharm. J. 2024, 32, 102041 10.1016/j.jsps.2024.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat M. T.; Ibrahim T. S.; Darwish K. M.; Khayyat A. N.; Alharbi M.; Khafagy E. S.; Ali M. A. M.; Hegazy W. A. H.; Abbas H. A. Hiring of the Anti-Quorum Sensing Activities of Hypoglycemic Agent Linagliptin to Alleviate the Pseudomonas aeruginosa Pathogenesis. Microorganisms 2022, 10 (12), 2455 10.3390/microorganisms10122455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alandiyjany M. N.; Abdelaziz A. S.; Abdelfattah-Hassan A.; Hegazy W. A. H.; Hassan A. A.; Elazab S. T.; Mohamed E. A. A.; El-Shetry E. S.; Saleh A. A.; ElSawy N. A.; Ibrahim D. Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals 2022, 15 (3), 357 10.3390/ph15030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. W.; Zhang Y.; Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1997, 94 (19), 10367–10372. 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rabia M. W.; Asfour H. Z.; Alhakamy N. A.; Bazuhair M. A.; Ibrahim T. S.; Abbas H. A.; Mansour B.; Hegazy W. A.; Seleem N. M. Cilostazol is a promising anti-pseudomonal virulence drug by disruption of quorum sensing. AMB Express 2024, 14 (1), 87 10.1186/s13568-024-01740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy W. A. H.; Salem I. M.; Alotaibi H. F.; Khafagy E.-S.; Ibrahim D. Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis. Antibiotics 2022, 11 (4), 465 10.3390/antibiotics11040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okairy H. M.; Koshak A. E.; Elfaky M. A.; Abdallah H. M.; Mohamed G. A.; Ibrahim S. R.; Alzain A. A.; Khafagy E.-S.; Rajab A. A.; Hegazy W. A. 6-Paradol exhibits antimicrobial, anti-quorum sensing and anti-virulence capacities on gram-negative bacteria: In vitro and in vivo studies. S. Afr. J. Bot. 2024, 174, 694–701. 10.1016/j.sajb.2024.09.034. [DOI] [Google Scholar]
- Koshak A. E.; Elfaky M. A.; Albadawi D. A. I.; Abdallah H. M.; Mohamed G. A.; Ibrahim S. R. M.; Alzain A. A.; Khafagy E. S.; Elsayed E. M.; Hegazy W. A. H.. Piceatannol: a renaissance in antibacterial innovation unveiling synergistic potency and virulence disruption against serious pathogens Int. Microbiol. 2024 10.1007/s10123-024-00532-8. [DOI] [PubMed]
- Elfaky M. A.; Koshak A. E.; Radwan M. F.; Abdallah H. M.; Mohamed G. A.; Ibrahim S. R. M.; Alzain A. A.; Rajab A. A. H.; Hegazy W. A. H. Honokiol from Magnolia Tree Exhibits Antibacterial and Anti-virulence Potential against Pseudomonas aeruginosa. Arabian J. Sci. Eng. 2024, 10.1007/s13369-024-09303-z. [DOI] [Google Scholar]
- Almalki A. J.; Ibrahim T. S.; Elhady S. S.; Darwish K. M.; Hegazy W. A. H. Repurposing α-Adrenoreceptor Blockers as Promising Anti-Virulence Agents in Gram-Negative Bacteria. Antibiotics 2022, 11 (2), 178 10.3390/antibiotics11020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabit A. K.; Eljaaly K.; Zawawi A.; Ibrahim T. S.; Eissa A. G.; Elbaramawi S. S.; Hegazy W. A. H.; Elfaky M. A. Muting Bacterial Communication: Evaluation of Prazosin Anti-Quorum Sensing Activities against Gram-Negative Bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens. Biology 2022, 11 (9), 1349 10.3390/biology11091349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach R. G.; Hensel M. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 2007, 120 (7–8), 317–327. [PubMed] [Google Scholar]
- Kuhle V.; Hensel M. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell. Mol. Life Sci. 2004, 61 (22), 2812–2826. 10.1007/s00018-004-4248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wael A. H. H.; Abbas H. A. Evaluation of the role of SsaV ‘Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr. J. Biotechnol. 2017, 16 (14), 718–726. 10.5897/AJB2016.15852. [DOI] [Google Scholar]
- Heggie A.; Cerny O.; Holden D. W. SteC and the intracellular Salmonella-induced F-actin meshwork. Cell. Microbiol. 2021, 23 (4), e13315 10.1111/cmi.13315. [DOI] [PubMed] [Google Scholar]; Poh J.; Odendall C.; Spanos A.; Boyle C.; Liu M.; Freemont P.; Holden D. W. SteC is a Salmonella kinase required for SPI-2-dependent F-actin remodelling. Cell. Microbiol. 2008, 10 (1), 20–30. 10.1111/j.1462-5822.2007.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuff K.; Finlay B. B. What the SIF Is Happening-The Role of Intracellular Salmonella-Induced Filaments. Front. Cell. Infect. Microbiol. 2017, 7, 335 10.3389/fcimb.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L. A.; Vallance B. A.; Hensel M.; Jäckel D.; Finlay B. B.; Steele-Mortimer O. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol. Microbiol. 2003, 49 (3), 685–704. 10.1046/j.1365-2958.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- Shapiro T. A.; Fahey J. W.; Wade K. L.; Stephenson K. K.; Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol., Biomarkers Prev. 2001, 10 (5), 501–508. [PubMed] [Google Scholar]