Abstract
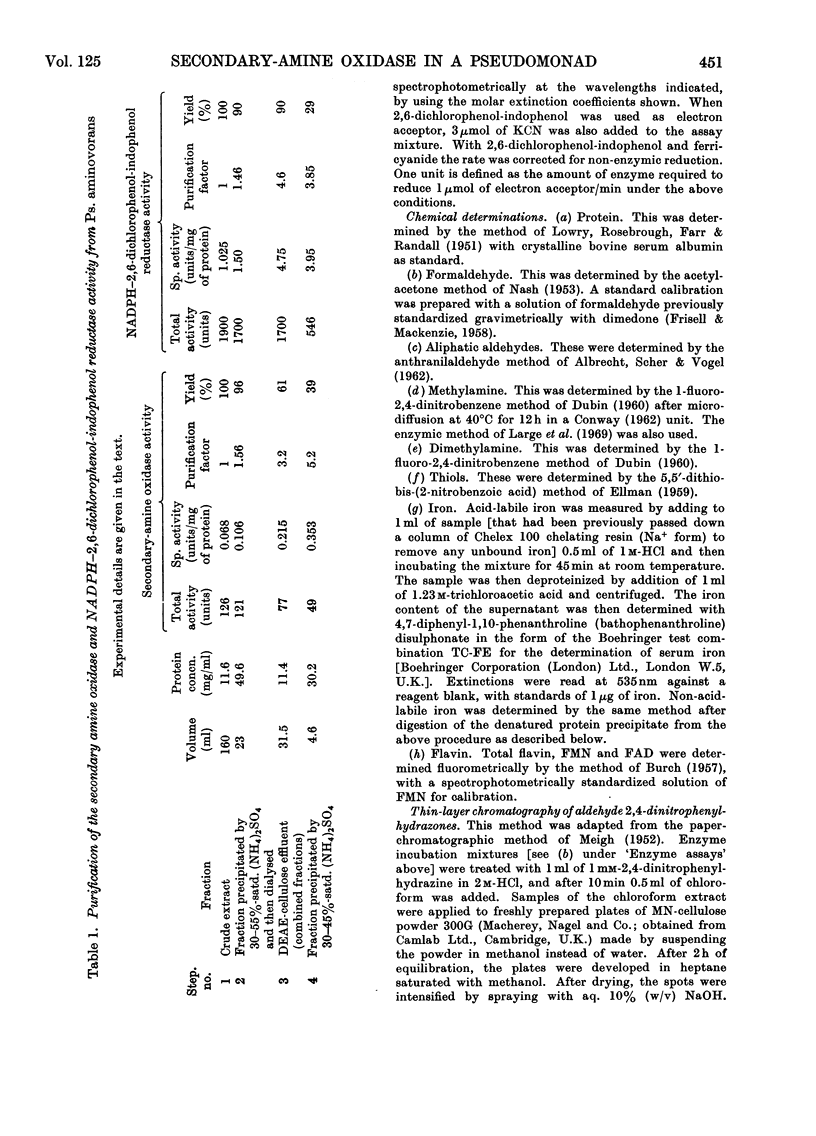
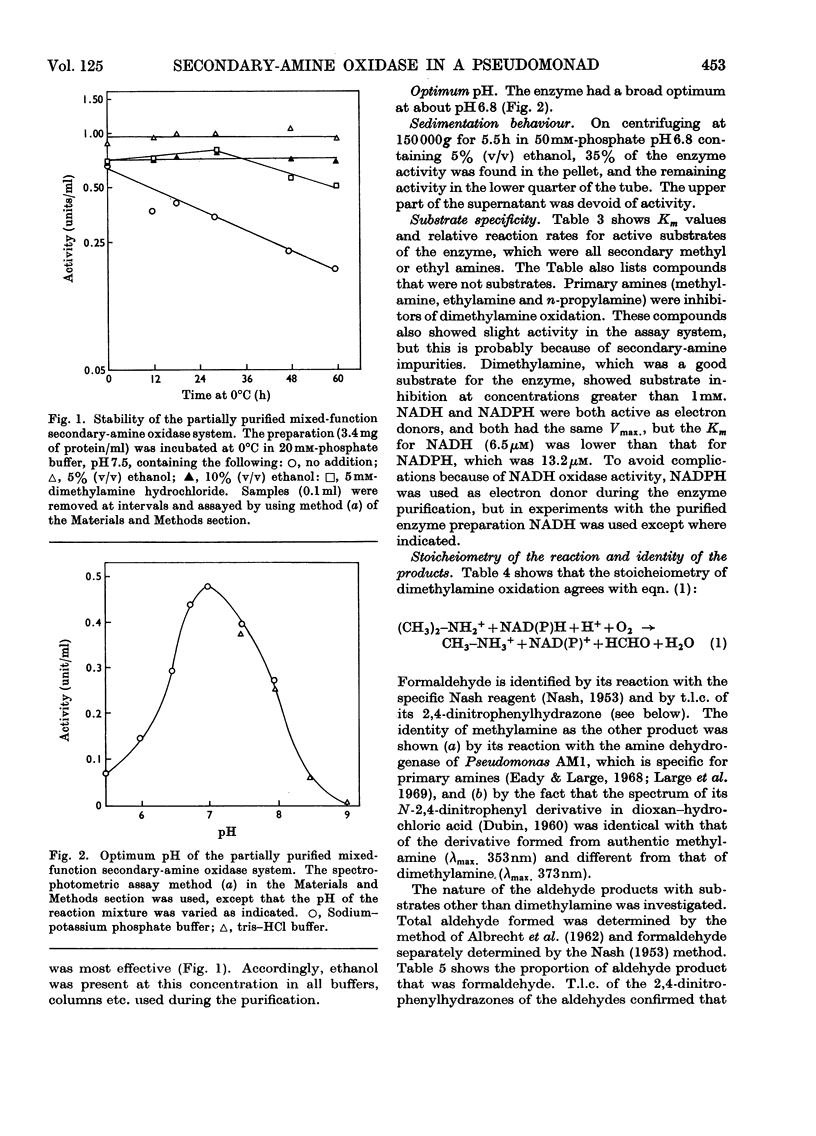
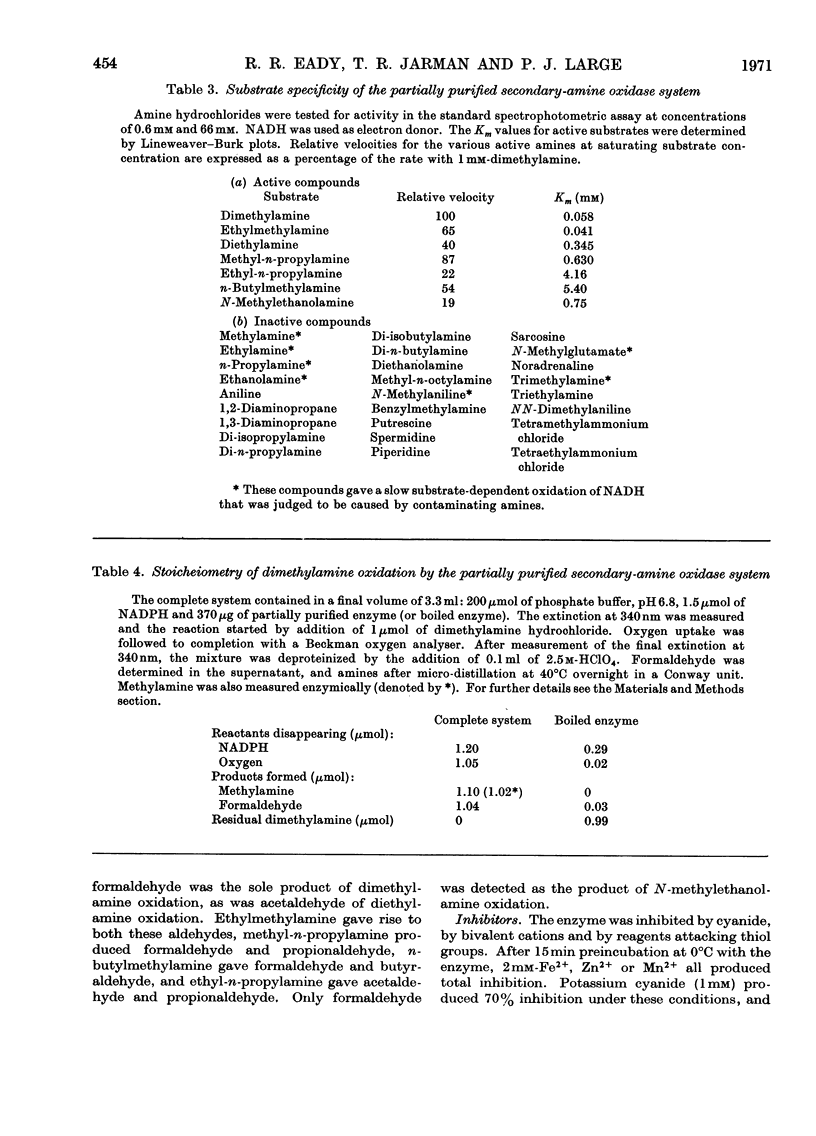
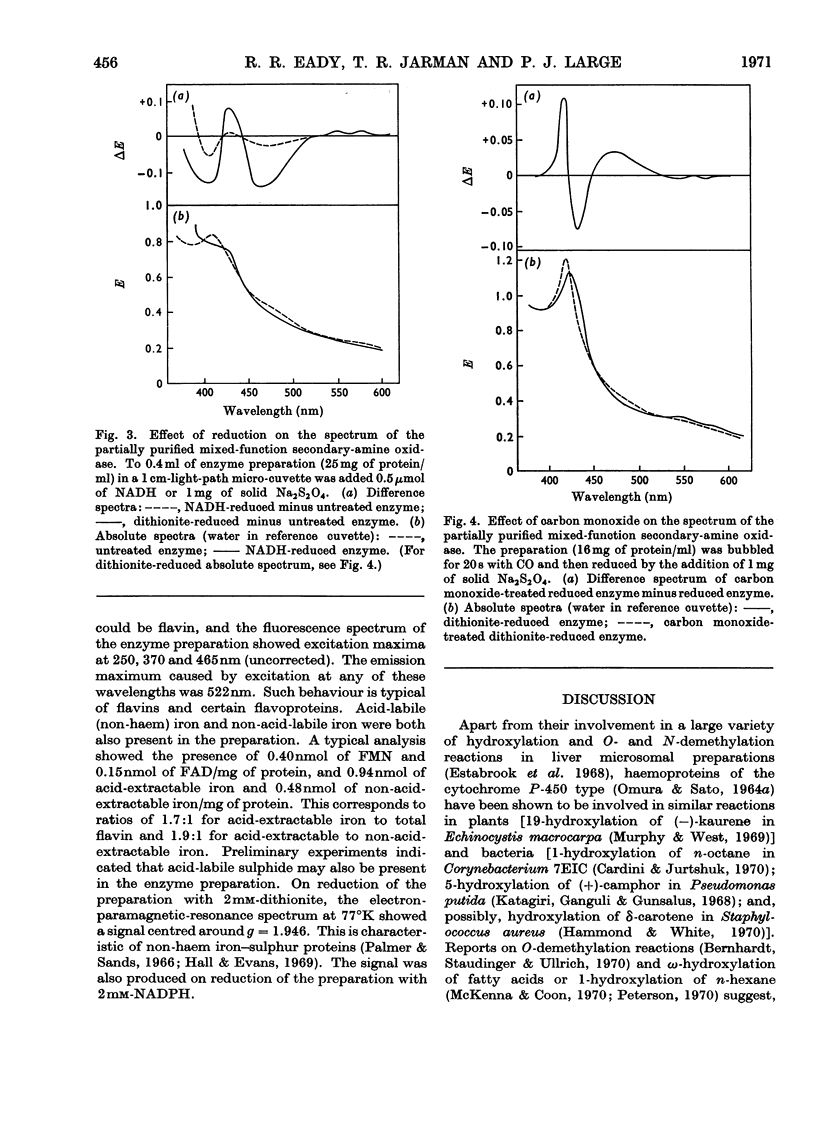
1. Crude extracts of Pseudomonas aminovorans grown on methylamine, di-methylamine, trimethylamine or trimethylamine N-oxide contain an enzyme or enzyme system catalysing the NADH- or NADPH- and oxygen-dependent oxidation of dimethylamine to methylamine and formaldehyde. 2. The enzyme has been partially purified about five-fold. It is unstable, but can be stabilized by addition of 5% (v/v) ethanol. 3. The partially purified enzyme will utilize either NADH (Km 6.5μm) or NADPH (Km 13.2μm): The following secondary amines have been shown to be substrates: dimethylamine, ethylmethylamine, diethylamine, methyl-n-propylamine, ethyl-n-propylamine, n-butylmethylamine and N-methylethanolamine. The Km values and comparative reaction rates for each substrate have been determined. Where the alkyl groups are different, the aldehyde products are derived from both groups. 4. The enzyme system has a pH optimum of 6.8 and is inhibited by mercurials, thiol compounds, cyanide and carbon monoxide. 5. The partially purified preparation had a spectral maximum at 412nm with shoulders at 427 and 550nm. Reduction with dithionite or NAD(P)H bleached the 412nm peak, and the shoulder at 427nm became a peak. Additional peaks appeared at 550 and 580–588nm. Reduction of a preparation bubbled with carbon monoxide enhanced and sharpened the Soret peak and caused it to shift to 422nm. 6. Analysis of the preparation showed the presence of flavin, acid-extractable iron and non-acid-extractable iron in the proportion 1.1:1.9:1. On reduction with dithionite or NADPH the preparation showed an electron-paramagnetic-resonance signal at around g=1.946.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhardt F. H., Staudinger H., Ullrich V. Eigenschaften einer p-Anisat-O-Demethylase im zellfreien Extrakt von Pseudomonas species. Hoppe Seylers Z Physiol Chem. 1970 Apr;351(4):467–478. [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Cardini G., Jurtshuk P. The enzymatic hydroxylation of n-octane by Corynebacterium sp. strain 7E1C. J Biol Chem. 1970 Jun 10;245(11):2789–2796. [PubMed] [Google Scholar]
- Colby J., Zatman L. J. The purification and properties of a bacterial trimethylamine dehydrogenase. Biochem J. 1971 Jan;121(1):9P–10P. doi: 10.1042/bj1210009p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T. The assay and characterization of amines by 2,4-dinitrofluorobenzene. J Biol Chem. 1960 Mar;235:783–786. [PubMed] [Google Scholar]
- Diehl H., Capalna S., Ullrich V. The photochemical action spectrum of the carbon monoxide inhibited hydroxylation of cyclohexane by rat liver microsomes. FEBS Lett. 1969 Jul;4(2):99–102. doi: 10.1016/0014-5793(69)80206-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Bacterial oxidation of dimethylamine, a new mono-oxygenase reaction. Biochem J. 1969 Mar;111(5):37P–38P. doi: 10.1042/bj1110037pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRI K. V., GOPALKRISHNAN, RADHAKRISHNAN, VAIDYANATHON Proline and hydroxproline in leaves. Nature. 1952 Oct 4;170(4327):579–580. doi: 10.1038/170579b0. [DOI] [PubMed] [Google Scholar]
- George H., McMahan J., Bowler K., Elliott M. Stabilization of lactate and malate dehydrogenase by organic solvents. Biochim Biophys Acta. 1969 Nov 4;191(2):466–468. doi: 10.1016/0005-2744(69)90266-6. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Evans M. C. Iron-sulphur proteins. Nature. 1969 Sep 27;223(5213):1342–1348. doi: 10.1038/2231342a0. [DOI] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Inhibition of carotenoid hydroxylation in Staphylococcus aureus by mixed-function oxidase inhibitors. J Bacteriol. 1970 Sep;103(3):607–610. doi: 10.1128/jb.103.3.607-610.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y., Yamano T., Fujishima H. Relationship between the interconversion of cytochrome P-450 and P-420 and its activities in hydroxylations and demethylations by P-450 oxidase systems. Biochim Biophys Acta. 1969 Jan 7;171(1):32–46. doi: 10.1016/0005-2744(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Kung H. F., Wagner C. Oxidation of C-1 compounds by Pseudomonas sp. MS. Biochem J. 1970 Feb;116(3):357–365. doi: 10.1042/bj1160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Eady R. R., Murden D. J. An enzymic method for the micro estimation of methylamine, ethylamine, and n-propylamine. Anal Biochem. 1969 Dec;32(3):402–407. doi: 10.1016/s0003-2697(69)80007-2. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Strobel H. W., Coon M. J. Properties of a solubilized form of the cytochrome P-450-containing mixed-function oxidase of liver microsomes. Mol Pharmacol. 1970 May;6(3):213–220. [PubMed] [Google Scholar]
- McKenna E. J., Coon M. J. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970 Aug 10;245(15):3882–3889. [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Palmer G., Sands R. H. On the magnetic resonance of spinach ferredoxin. J Biol Chem. 1966 Jan 10;241(1):253–253. [PubMed] [Google Scholar]
- Peterson J. A., Basu D., Coon M. J. Enzymatic omega-oxidation. I. Electon carriers in fatty acid and hydrocarbon hydroxylation. J Biol Chem. 1966 Nov 10;241(21):5162–5164. [PubMed] [Google Scholar]
- Peterson J. A., Coon M. J. Enzymatic omega-oxidation. 3. Purification and properties of rubredoxin, a component of the omega-hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1968 Jan 25;243(2):329–334. [PubMed] [Google Scholar]
- Peterson J. A. Cytochrome content of two pseudomonads containing mixed-function oxidase systems. J Bacteriol. 1970 Sep;103(3):714–721. doi: 10.1128/jb.103.3.714-721.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsin B., Brodie A. F. Carbon monoxide-binding pigments of Mycobacterium phlei and Escherichia coli. J Biol Chem. 1969 Jun 10;244(11):3101–3104. [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. The purification and properties of benzylalcohol dehydrogenase from Pseudomonas sp. Arch Biochem Biophys. 1969 Mar;130(1):422–429. doi: 10.1016/0003-9861(69)90054-x. [DOI] [PubMed] [Google Scholar]
- Wilson L. D., Harding B. W. Studies on adrenal cortical cytochrome P-450. 3. Effects of carbon monoxide and light on steroid 11-beta hydroxylation. Biochemistry. 1970 Mar 31;9(7):1615–1621. doi: 10.1021/bi00809a021. [DOI] [PubMed] [Google Scholar]


