Abstract
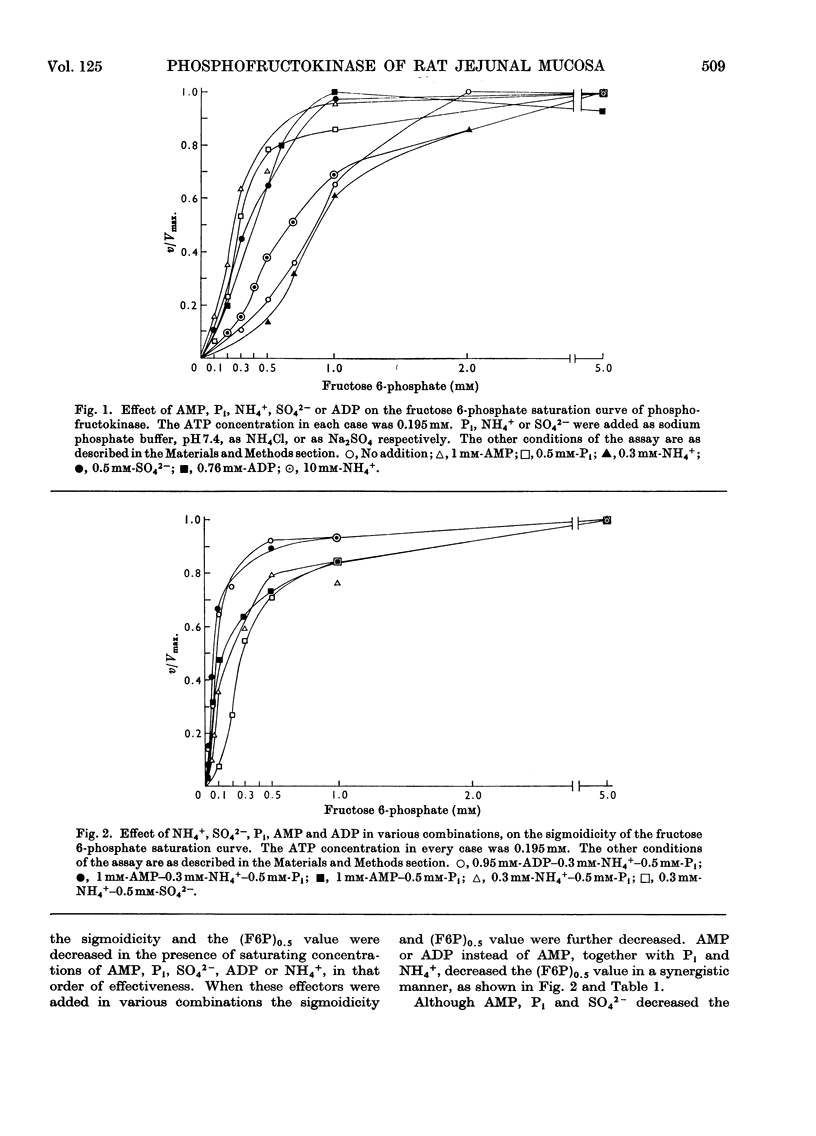
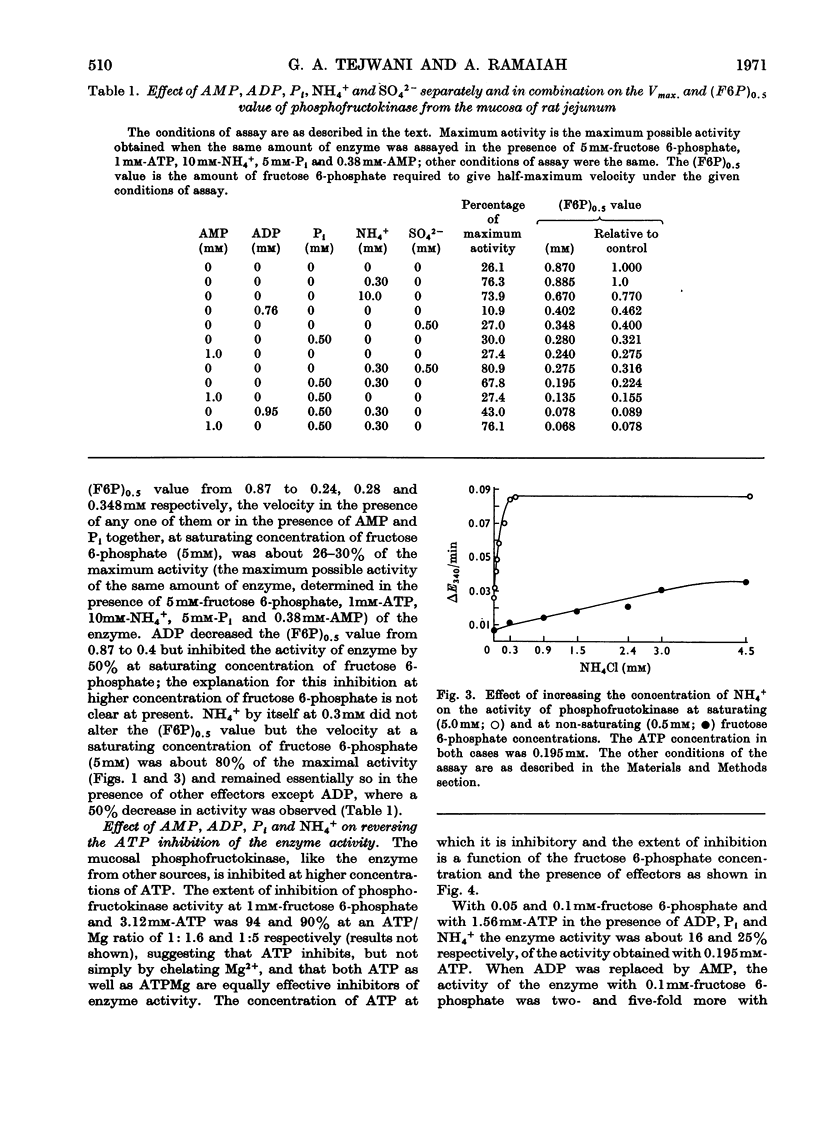
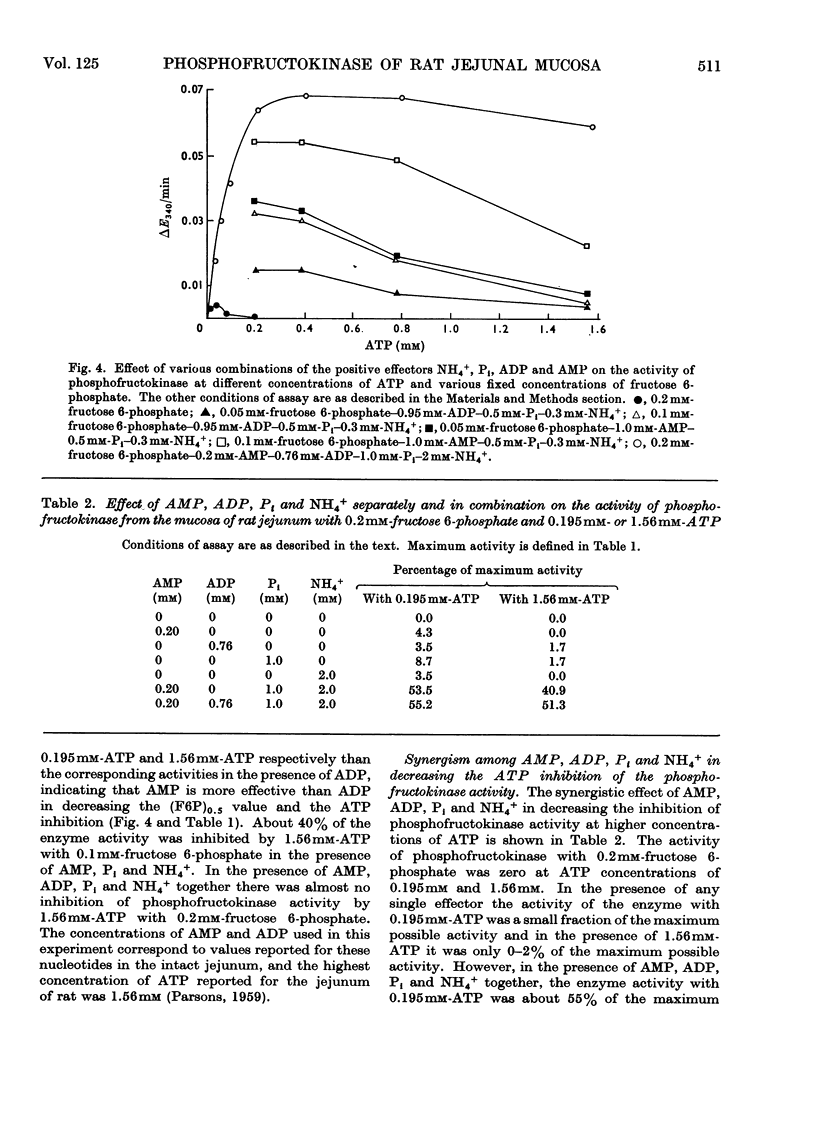
1. The properties of phosphofructokinase after its slight purification from the mucosa of rat jejunum were studied. 2. The enzyme is inhibited by almost 100% by an excess of ATP (1.6mm), with 0.2mm-fructose 6-phosphate. AMP, ADP, Pi and NH4+ at 0.2, 0.76, 1.0 and 2mm respectively do not individually prevent the inhibition of phosphofructokinase activity by 1.6mm-ATP with 0.2mm-fructose 6-phosphate to any great extent, but all of them together completely prevent the inhibition of phosphofructokinase by ATP. 3. One of the effects of high concentrations of ATP on the enzyme was to increase enormously the apparent Km value for the other substrate fructose 6-phosphate, and this increase is largely counteracted by the presence of AMP, ADP, Pi and NH4+. At low concentrations of ATP the above effectors individually decrease the concentration of fructose 6-phosphate required for half-maximum velocity and when present together they decrease it further, in a more than additive way. 4. When fructose 6-phosphate is present at a saturating concentration (5mm), 0.3mm-NH4+ increases the maximum velocity of the reaction 3.3-fold; with 0.5mm-fructose 6-phosphate, 4.5mm-NH4+ is required for maximum effect. The other effectors do not change the maximum reaction velocity. 5. The results presented here suggest that NH4+, AMP, ADP and Pi synergistically decrease the inhibition of phosphofructokinase activity at high concentrations of ATP by decreasing the concentration of fructose 6-phosphate required for half-maximum velocity. Such synergism among the effectors and an observed, low `energy charge' [(ATP+½ADP)/(AMP+ADP+ATP)] in conjunction with the possibility of a relatively high NH4+ and fructose 6-phosphate concentration in this tissue, may keep the mucosal phosphofructokinase active and uninhibited by ATP under aerobic conditions, thus explaining the high rate of aerobic glycolysis and the lack of Pasteur effect in this tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- BROWN R. H., DUDA G. D., KORKES S., HANDLER P. A colorimetric micromethod for determination of ammonia; the ammonia content of rat tissues and human plasma. Arch Biochem Biophys. 1957 Feb;66(2):301–309. doi: 10.1016/s0003-9861(57)80005-8. [DOI] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: The metabolism of intestinal mucous membrane. Biochem J. 1941 Jan;35(1-2):7–15. doi: 10.1042/bj0350007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELE M. P. The phosphorylation and absorption of sugars in the rat. 2. Sugar absorption in vivo, and its relationship to the phosphorylation of sugar in vitro. Biochem J. 1953 Dec;55(5):864–867. doi: 10.1042/bj0550864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemhoff W. G., van den Berg J. W., de Pijper A. M., Hülsmann W. C. Metabolic aspects of isolated cells from rat small intestinal epithelium. Biochim Biophys Acta. 1970 Aug 14;215(2):229–241. doi: 10.1016/0304-4165(70)90020-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Passonneau J. V. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966 May 25;241(10):2268–2279. [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Sugden P. H. The apparent inhibition of phosphofrunctokinase by reduced nicotinamide-adenine dinucleotide: a problem of coupled-enzyme assays. Biochem J. 1970 Oct;119(4):787–789. doi: 10.1042/bj1190787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPADOPOULOS N. M., ROE J. H. Fructose phosphorylation and dephosphorylation by the intestinal mucosa of the rat during fructose absorption. Am J Physiol. 1957 May;189(2):301–306. doi: 10.1152/ajplegacy.1957.189.2.301. [DOI] [PubMed] [Google Scholar]
- PARKER V. H. The effect of 3:5-dinitroortho-cresol on phosphocreatine and the adenosine phosphate compounds of rat tissues. Biochem J. 1954 Jul;57(3):381–386. doi: 10.1042/bj0570381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS B. J. Studies of the effect of triethyltin sulphate on transport and metabolism in the small intestine of the rat. J Physiol. 1959 Oct;148:117–126. doi: 10.1113/jphysiol.1959.sp006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- RAMAIAH A., HATHAWAY J. A., ATKINSON D. E. ADENYLATE AS A METABOLIC REGULATOR. EFFECT ON YEAST PHOSPHOFRUCTOKINASE KINETICS. J Biol Chem. 1964 Nov;239:3619–3622. [PubMed] [Google Scholar]
- REYNELL P. C., SPRAY G. H. The absorption of glucose by the intact rat. J Physiol. 1956 Dec 28;134(3):531–537. doi: 10.1113/jphysiol.1956.sp005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS M. L., VINUELA E., SALAS M., SOLS A. CITRATE INHIBITION OF PHOSPHOFRUCTOKINASE AND THE PASTEUR EFFECT. Biochem Biophys Res Commun. 1965 Apr 23;19:371–376. doi: 10.1016/0006-291x(65)90471-7. [DOI] [PubMed] [Google Scholar]
- Shen L. C., Fall L., Walton G. M., Atkinson D. E. Interaction between energy charge and metabolite modulation in the regulation of enzymes of amphibolic sequences. Phosphofructokinase and pyruvate dehydrogenase. Biochemistry. 1968 Nov;7(11):4041–4045. doi: 10.1021/bi00851a035. [DOI] [PubMed] [Google Scholar]
- Srivastava L. M., Hübscher G. Glucose metabolism in the mucosa of the small intestine. Glycolysis in subcellular preparations from the cat and rat. Biochem J. 1966 Aug;100(2):458–466. doi: 10.1042/bj1000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerskill W. H., Aoyagi T., Evans W. B. Ammonia in the upper gastrointestinal tract of man: quantitations and relationships. Gut. 1966 Oct;7(5):497–501. doi: 10.1136/gut.7.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. Metabolic activity of the small intestine of the rat and golden hamster (Mesocricetus auratus). J Physiol. 1954 Jan;123(1):126–130. doi: 10.1113/jphysiol.1954.sp005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]


