Abstract
Background
Hypertension (HTN) is a global public health concern and a major risk factor for cardiovascular disease (CVD) and mortality. Insulin resistance (IR) plays a crucial role in HTN-related metabolic dysfunction, but its assessment remains challenging. The triglyceride–glucose (TyG) index and its derivatives (TyG–BMI, TyG–WC, and TyG–WHtR) have emerged as reliable IR markers. In this study, we evaluated their associations with all-cause and cardiovascular mortality in hypertensive patients using machine learning techniques.
Methods
Data from 9432 hypertensive participants in the National Health and Nutrition Examination Survey (NHANES) 1999–2018 were analysed. Cox proportional hazards models and restricted cubic splines were employed to explore mortality risk and potential nonlinear relationships. Machine learning models were utilized to assess the predictive value of the TyG index and its derivatives for mortality outcomes.
Results
The TyG index and its derivatives were independent predictors of both all-cause and cardiovascular mortality in hypertensive patients. The TyG–WHtR exhibited the strongest association, with each 1-unit increase linked to a 41.7% and 48.1% higher risk of all-cause and cardiovascular mortality, respectively. L-shaped relationships were observed between TyG-related indices and mortality. The incorporation of the TyG index or its derivatives into predictive models modestly improved the prediction performance for mortality outcomes.
Conclusions
The TyG index and its derivatives are significant predictors of mortality in hypertensive patients. Their inclusion in predictive models enhances risk stratification and may aid in the early identification of high-risk individuals in this population. Further studies are needed to validate these findings in external hypertensive cohorts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02591-1.
Keywords: Triglyceride–glucose (TyG) index, Hypertension, Mortality, Machine learning, National Health and Nutrition Examination Survey (NHANES)
Background
Hypertension (HTN) is a prevalent chronic disease and a leading risk factor for cardiovascular and cerebrovascular events. Despite global efforts, HTN control remains a significant public health challenge. From 1990 to 2019, the global prevalence of HTN doubled from 650 million to 1.3 billion individuals [1]. By 2025, the prevalence of HTN among adults is projected to reach 29.2%, which is expected to be driven primarily by population ageing [2]. Among adults with HTN aged 30–79 years, approximately 42% receive antihypertensive treatment, but only 21% achieve adequate blood pressure control [3]. In the United States, the average annual direct medical cost per patient with HTN is $5768, contributing to a national healthcare expenditure of $173 billion. Globally, HTN-related healthcare costs range from $131 billion to $198 billion annually, imposing a substantial economic burden [4–6]. Early identification and intervention in high-risk HTN patients may reduce adverse outcomes and provide a cost-effective management strategy.
The pathophysiology of HTN is closely linked to metabolic abnormalities, with insulin resistance (IR) playing a pivotal role. IR is characterized by a diminished tissue response to insulin, resulting in impaired glucose uptake, hyperglycaemia, and various metabolic disorders [7]. IR has been significantly associated with increased cardiovascular disease (CVD) risk [8–10]. However, traditional IR assessment methods, such as the hyperinsulinaemic-euglycaemic clamp, are complex and costly, making them impractical for large-scale screening. Additionally, no universally accepted IR metrics exist for hypertensive populations, limiting the ability to conduct broader research and develop clinical applications. The triglyceride–glucose (TyG) index has emerged as a reliable alternative marker for IR, demonstrating superior sensitivity and specificity in clinical and epidemiological studies [11]. To increase its predictive value for CVD risk, derivatives of the TyG index incorporating obesity-related metrics, such as body mass index (BMI), waist circumference (WC), and the waist-to-height ratio (WHtR), have been developed (TyG–BMI, TyG–WC, TyG–WHtR). Zhu et al. [12] reported that TyG–WC exhibited the strongest association with CVD risk, whereas Ren et al. [13] reported that moderate or high TyG–WHtR was significantly associated with increased CVD risk after adjusting for confounders. These indices may serve as effective predictors of CVD risk.
Although the TyG index and its derivatives have been implicated in the pathophysiology of HTN, studies investigating their associations with mortality outcomes remain limited. Furthermore, the potential of machine learning techniques to evaluate the relationships among the TyG index, its derivatives, and long-term mortality in hypertensive populations has yet to be fully explored. In this study, we sought to address these knowledge gaps by applying machine learning approaches to assess the predictive value of the TyG index and its obesity-related derivatives for all-cause and cardiovascular mortality in hypertensive patients, with the aim of improving risk stratification and informing strategies for adverse outcome prevention in this high-risk population.
Methods
Study design and population
In the present study, we utilized data from the National Health and Nutrition Examination Survey (NHANES), which was conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). Details of the NHANES study design and methodology have been previously described [14]. The study protocols were approved by the NCHS Research Ethics Review Board, and all participants provided written informed consent. We conducted this study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Data from 10 survey cycles (1999–2018) were retrieved from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The inclusion criteria were as follows: (1) all participants aged ≥ 20 years from NHANES 1999–2018 with diagnosed HTN. The exclusion criteria were as follows: (1) missing fasting plasma glucose (FPG) data; (2) missing triglyceride (TG) data; (3) missing BMI data; (4) missing WC data; and (5) missing follow-up data. Blood pressure measurements were averaged from three readings. HTN was defined as a mean systolic blood pressure (SBP) ≥ 140 mmHg, mean diastolic blood pressure (DBP) ≥ 90 mmHg, or current use of antihypertensive medication. A total of 9432 eligible participants were included. The selection process is illustrated in Supplementary Fig. 1.
Assessment of the TyG index and its combined obesity indices
The TyG index was calculated as follows [15]:
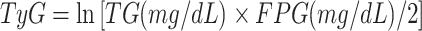 |
The combined obesity indices were defined as follows [16, 17]:
 |
 |
 |
The participants were categorized into quartiles (Q1, Q2, Q3, and Q4) for each index, with Q1 serving as the reference group.
Assessment of mortality
Mortality data were linked to the National Death Index (NDI), which provides cause-of-death information. The follow-up period was calculated from the baseline interview to either the date of death or December 31, 2019. The primary outcomes included all-cause and cardiovascular mortality. All-cause mortality was defined as death from any cause, including heart disease, malignant neoplasms, chronic lower respiratory diseases, unintentional injuries, cerebrovascular diseases, Alzheimer’s disease, diabetes mellitus (DM), influenza or pneumonia, kidney disease, or other causes. Cardiovascular mortality included deaths from heart disease or cerebrovascular disease, coded as UCOD_LEADING = 001 or 005.
Covariates
Baseline sociodemographic and health-related data were collected via structured interviews. The variables included age, sex, race, marital status, education level, BMI, WC, SBP, DBP, smoking status, alcohol consumption status, and comorbidities [coronary heart disease (CHD), angina, congestive heart failure (CHF), stroke, and DM]. The laboratory variables included FPG, glycosylated haemoglobin (HbA1c), total cholesterol (TC), TG, albumin (ALB), white blood cell (WBC) count, haemoglobin (Hb), C-reactive protein (CRP), and serum creatinine. The estimated glomerular filtration rate (eGFR) was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation [18].
Statistical analysis
Baseline characteristics were stratified by quartiles of the TyG index and its combined obesity indices. Continuous variables are presented as the means ± standard deviations, whereas categorical variables are expressed as frequency counts and percentages. P values for continuous variables were calculated using one-way ANOVA, and Pearson’s chi-square test was used for categorical variables. Missing data were imputed using multiple imputation methods. The statistical analyses incorporated sample weights, clustering, and stratification due to the complex multistage stratified probability survey design employed in the NHANES. Mortality outcomes were assessed via Kaplan–Meier (K–M) survival curves and log-rank tests. Cox proportional hazards models were used to evaluate the prognostic significance of the TyG index and its combined obesity indices. Three Cox models were constructed with different levels of adjustment: Model 1 was unadjusted; Model 2 was adjusted for age, sex, race, smoking status, alcohol consumption status, and BMI; and Model 3 was adjusted for age, sex, race, smoking status, alcohol consumption status, BMI, stroke status, DM status, TC levels, and the eGFR. Restricted cubic spline (RCS) analysis was performed to assess nonlinear relationships of the TyG index and its combined obesity indices with all-cause and cardiovascular mortality. The number of knots was set to 3.
The state-of-the-art machine learning techniques were used to further address the variable covariance and its effect on the results. To simplify the machine learning models, we performed feature selection to identify the predictor variables most important for outcome prediction. First, predictor variables with near-zero variance (< 5% unique value) were eliminated. Two feature selection methods—least absolute shrinkage and selection operator (LASSO) and the Boruta algorithm—were subsequently used to identify significant predictors for machine learning analysis. The results from both methods were combined, and variables that appeared consistently were selected for constructing the basic model. The final basic model variables included were age, BMI, CHF status, CRP levels, the eGFR, education level, mean SBP, smoking status, TC levels, and the WBC count. Feature selection was performed using the Glmnet, Boruta, and Caret packages in R.
The following machine learning algorithms were applied to the dataset after variable selection, and the combined dataset was used for model development: logistic regression, k-nearest neighbours (K-NN), naive Bayes, support vector machines (SVMs), random forests, eXtreme Gradient Boosting (XGBoost), and neural networks. Fivefold cross-validation with five random shufflings (5 × 5) was used to evaluate model performance and determine the best hyperparameters. Model performance was evaluated using the mean area under the receiver operating characteristic curve (AUROC) across the five folds. The best-performing machine learning model for each dataset was selected on the basis of its AUROC. The caret package in R was used for model development. Details of the functions, packages, and tuning parameters for each machine learning algorithm are provided in Supplementary Table 1. The best-performing machine learning algorithm was then applied to assess the performance of the basic model and four optimized models incorporating the TyG index, TyG–BMI, TyG–WC, and TyG–WHtR by comparing their concordance statistics (C statistics). The differences in C statistics were assessed using the bootstrap method. All the statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing), along with Zstats v0.90 (www.medsta.cn/software). A P value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of the participants
A total of 9432 hypertensive participants were included in the study. The baseline characteristics stratified by all-cause and cardiovascular death outcomes are presented in Table 1. Compared with survivors, participants who died from all-cause or cardiovascular events were more likely to be older, male, and non-Hispanic White and to smoke and have a lower BMI. Among hypertensive patients who experienced all-cause or cardiovascular death, DM was the most prevalent comorbidity, with rates of 33.81% and 35.61%, respectively. Significant differences in the TyG index and TyG–BMI were observed between groups stratified by outcome. Hypertensive patients who died had higher TyG indices but lower TyG–BMI values. Baseline characteristics stratified by quartiles of the TyG index and its derivatives are detailed in Supplementary Tables 2–5.
Table 1.
Characteristics of HTN patients according to the presence of both all-cause and cardiovascular mortality
| All-cause mortality | Cardiovascular mortality | |||||
|---|---|---|---|---|---|---|
| Survivor | Non-Survivor | P value | Survivor | Non-Survivor | P value | |
| N = 7084 | N = 2348 | N = 8631 | N = 801 | |||
| Age (y) | 53.97 (0.23) | 68.11 (0.40) | < 0.001 | 55.81 (0.23) | 69.58 (0.58) | < 0.001 |
| Sex (n, %) | 0.329 | 0.040 | ||||
| Male | 3386 (49.13) | 1256 (50.49) | 4202 (49.12) | 440 (53.57) | ||
| Female | 3698 (50.87) | 1092 (49.51) | 4429 (50.88) | 361 (46.43) | ||
| Marital status (n, %) | < 0.001 | < 0.001 | ||||
| Married | 4703 (68.62) | 1829 (78.19) | 5901 (69.92) | 631 (78.38) | ||
| Divorced | 1129 (14.30) | 322 (12.85) | 1345 (14.10) | 106 (12.94) | ||
| Never married | 1252 (17.07) | 197 (8.97) | 1385 (15.98) | 64 (8.68) | ||
| Race (n, %) | < 0.001 | 0.001 | ||||
| Mexican American | 1060 (5.99) | 301 (3.30) | 1265 (5.64) | 96 (3.16) | ||
| Other Hispanic | 618 (4.73) | 100 (3.09) | 684 (4.50) | 34 (3.09) | ||
| Non-Hispanic White | 2942 (69.38) | 1402 (78.14) | 3878 (70.73) | 466 (75.94) | ||
| Non-Hispanic Black | 1821 (13.12) | 471 (11.59) | 2115 (12.76) | 177 (13.79) | ||
| Other races | 643 (6.78) | 74 (3.89) | 689 (6.37) | 28 (4.02) | ||
| Education level (n, %) | < 0.001 | < 0.001 | ||||
| Less than high school | 1929 (17.25) | 923 (29.51) | 2540 (18.87) | 312 (30.35) | ||
| High school or equivalent | 1718 (25.91) | 611 (29.81) | 2124 (26.52) | 205 (28.69) | ||
| College or above | 3437 (56.85) | 814 (40.68) | 3967 (54.61) | 284 (40.96) | ||
| Alcohol consumption status (n, %) | 0.003 | 0.001 | ||||
| < 5 drinks/year | 6269 (89.09) | 2152 (91.84) | 7677 (89.33) | 744 (93.85) | ||
| 5–9 drinks/year | 628 (8.36) | 160 (6.84) | 744 (8.27) | 44 (5.05) | ||
| ≥ 10 drinks/year | 187 (2.55) | 36 (1.33) | 210 (2.40) | 13 (1.10) | ||
| Smoking status (n, %) | < 0.001 | 0.072 | ||||
| No | 3723 (50.78) | 962 (39.55) | 4326 (48.90) | 359 (44.73) | ||
| Yes | 3361 (49.22) | 1386 (60.45) | 4305 (51.10) | 442 (55.27) | ||
| Mean SBP (mmHg) | 132.30 (0.31) | 140.02 (0.62) | < 0.001 | 133.21 (0.29) | 142.18 (1.01) | < 0.001 |
| Mean DBP (mmHg) | 74.48 (0.28) | 67.83 (0.44) | < 0.001 | 73.62 (0.26) | 67.13 (0.77) | < 0.001 |
| Height (cm) | 168.71 (0.16) | 166.72 (0.27) | < 0.001 | 168.46 (0.16) | 166.47 (0.46) | < 0.001 |
| Weight (kg) | 89.02 (0.36) | 81.22 (0.65) | < 0.001 | 87.89 (0.32) | 82.13 (1.12) | < 0.001 |
| BMI (kg/m2) | 31.17 (0.12) | 29.04 (0.19) | < 0.001 | 30.85 (0.11) | 29.45 (0.35) | < 0.001 |
| WC (cm) | 105.14 (0.28) | 103.06 (0.51) | < 0.001 | 104.79 (0.25) | 103.99 (0.81) | 0.349 |
| Stroke (n, %) | 353 (4.10) | 289 (11.57) | < 0.001 | 527 (5.03) | 115 (13.03) | < 0.001 |
| DM (n, %) | 1900 (21.18) | 835 (33.81) | < 0.001 | 2442 (22.79) | 293 (35.61) | < 0.001 |
| CHD (n, %) | 403 (5.35) | 315 (14.21) | < 0.001 | 586 (6.31) | 132 (18.03) | < 0.001 |
| CHF (n, %) | 292 (3.35) | 280 (11.82) | < 0.001 | 460 (4.35) | 112 (14.21) | < 0.001 |
| Angina (n, %) | 275 (3.82) | 223 (10.05) | < 0.001 | 397 (4.46) | 101 (13.21) | < 0.001 |
| FPG (mg/dL) | 112.05 (0.61) | 121.30 (1.31) | < 0.001 | 113.10 (0.59) | 124.68 (2.42) | < 0.001 |
| HbA1c (%) | 5.81 (0.02) | 6.03 (0.03) | < 0.001 | 5.83 (0.02) | 6.17 (0.06) | < 0.001 |
| TC (mg/dL) | 198.46 (0.73) | 196.68 (1.04) | 0.135 | 198.25 (0.65) | 196.22 (2.09) | 0.323 |
| TG (mg/dL) | 150.63 (2.21) | 151.24 (2.67) | 0.857 | 150.81 (1.99) | 149.78 (4.08) | 0.819 |
| ALB (g/dL) | 4.21 (0.01) | 4.15 (0.01) | < 0.001 | 4.20 (0.01) | 4.14 (0.02) | < 0.001 |
| WBC (× 109/L) | 6.94 (0.04) | 7.33 (0.08) | < 0.001 | 7.00 (0.04) | 7.20 (0.09) | 0.048 |
| Hb (g/dL) | 14.41 (0.03) | 14.12 (0.05) | < 0.001 | 14.37 (0.03) | 14.10 (0.06) | < 0.001 |
| CRP (mg/dL) | 0.55 (0.02) | 0.67 (0.03) | 0.002 | 0.57 (0.02) | 0.66 (0.05) | 0.122 |
| eGFR (mL/min/1.73m2) | 87.67 (0.41) | 73.06 (0.68) | < 0.001 | 85.79 (0.38) | 71.20 (1.15) | < 0.001 |
| TyG index | 8.83 (0.01) | 8.91 (0.02) | < 0.001 | 8.84 (0.01) | 8.94 (0.03) | 0.009 |
| TyG–BMI | 276.17 (1.27) | 260.05 (2.04) | < 0.001 | 273.67 (1.15) | 264.39 (3.56) | 0.015 |
| TyG–WC | 931.24 (3.48) | 921.65 (5.66) | 0.162 | 929.22 (3.12) | 932.33 (8.90) | 0.745 |
| TyG–WHtR | 5.53 (0.02) | 5.53 (0.03) | 0.881 | 5.52 (0.02) | 5.60 (0.05) | 0.135 |
Bold values indicate statistical significance. A P value < 0.05 was considered to indicate statistical significance
ALB Albumin; BMI Body mass index; CHD Coronary heart disease; CHF Chronic heart failure; CRP C-reactive protein; DBP Diastolic blood pressure; DM Diabetes mellitus; eGFR Estimated glomerular filtration rate; FPG Fasting plasma glucose; Hb Haemoglobin; HbA1c Glycosylated haemoglobin; HTN Hypertension; SBP systolic blood pressure; TC Total cholesterol; TG Triglyceride; TyG Triglyceride–glucose; WBC White blood cell; WC Waist circumference; WHtR Waist-to-height ratio
Clinical outcomes for all-cause and cardiovascular mortality
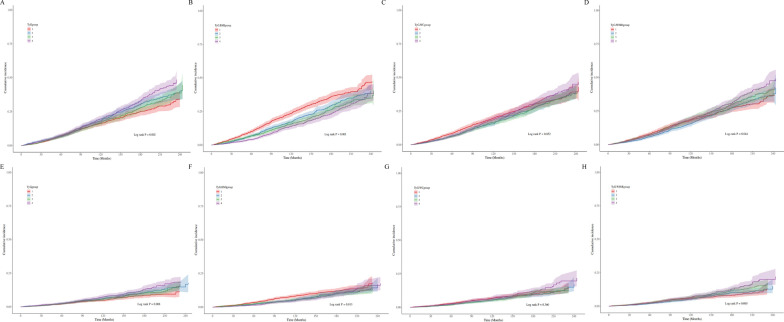
During a mean follow-up period of 110 ± 1.4 months, there were 2348 cases of all-cause mortality and 801 cases of cardiovascular mortality among hypertensive patients. The all-cause mortality rate was 27.1 per 1000 person-years, whereas the cardiovascular mortality rate was 9.3 per 1000 person-years. K–M survival analysis revealed significant differences in all-cause mortality rates across quartiles of the TyG index, TyG–BMI, and TyG–WHtR. Specifically, the highest all-cause mortality rates were observed in Q4 of the TyG index, Q1 of TyG–BMI, and Q4 of TyG–WHtR (log-rank P TyG = 0.002; log-rank P TyG–BMI < 0.001; log-rank P TyG–WHtR = 0.044, respectively). Similar patterns were observed in cardiovascular mortality across these quartiles (Fig. 1).
Fig. 1.
K–M analyses for all-cause (a, b, c, and d) and cardiovascular mortality (e, f, g, and h) among the TyG index and its derivatives. BMI, Body mass index; K–M, Kaplan–Meier; TyG, Triglyceride‒glucose; WC, Waist circumference; WHtR, Waist-to-height ratio
The Cox regression models used to assess the associations of the TyG index and its derived indices with all-cause mortality are presented in Table 2. The TyG index, as a continuous variable, was significantly associated with all-cause mortality in both the unadjusted and adjusted models. In the fully adjusted Model 3, each 1-unit increase in the TyG index was associated with a 13.1% higher risk of all-cause mortality (hazard ratio [HR]: 1.131, 95% confidence interval [CI]: 1.021–1.252, P = 0.018). When the data were stratified by quartiles, Q2 and Q4 were positively associated with all-cause mortality in the unadjusted model, but this significance disappeared after covariate adjustment. In contrast, the TyG–BMI was significantly associated with all-cause mortality both before and after adjustment, with Q2 demonstrating a protective effect (HR: 0.802, 95% CI: 0.673–0.956; P = 0.014). TyG–WC, as a continuous variable, was not associated with all-cause mortality in the unadjusted model (P = 0.834) but became significant after adjustment (HR: 1.002, 95% CI 1.001–1.002; P < 0.001). The Q4 of TyG–WC also showed a significant positive association with all-cause mortality after adjusting for covariates (HR: 1.435, 95% CI 1.055–1.952; P = 0.022). The TyG–WHtR exhibited the strongest association with all-cause mortality, with each 1-unit increase associated with a 41.7% increased risk (P < 0.001). Similarly, the Q4 of the TyG–WHtR remained significantly associated with all-cause mortality after adjustment (HR: 1.677, 95% CI 1.288–2.184; P < 0.001).
Table 2.
Cox regression models for the associations between TyG-related indices and mortality in patients with HTN
| Indices | Groups | Number | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|
| HR (95% CI) P value | HR (95% CI) P value | HR (95% CI) P value | |||
| All-cause mortality | |||||
| TyG index | Continuous | N = 9432 | 1.195 (1.095–1.304) < 0.001 | 1.244 (1.134–1.364) < 0.001 | 1.131 (1.021–1.252) 0.018 |
| Q1 (< 8.39) | N = 2461 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [8.39–8.79) | N = 2338 | 1.204 (1.014–1.428) 0.034 | 1.053 (0.921–1.204) 0.448 | 1.017 (0.885–1.168) 0.817 | |
| Q3 [8.79–9.23) | N = 2310 | 1.104 (0.926–1.318) 0.270 | 1.023 (0.876–1.196) 0.773 | 0.937 (0.792–1.108) 0.444 | |
| Q4 (≥ 9.23) | N = 2323 | 1.347 (1.141–1.589) < 0.001 | 1.312 (1.112–1.549) 0.001 | 1.111 (0.928–1.331) 0.251 | |
| P for trend | 0.002 | 0.003 | 0.418 | ||
| TyG–BMI | Continuous | N = 9432 | 0.997 (0.996–0.998) < 0.001 | 1.008 (1.005–1.011) < 0.001 | 1.005 (1.002–1.009) 0.002 |
| Q1 (< 223.94) | N = 2431 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [223.94–263.44) | N = 2481 | 0.755 (0.651–0.875) < 0.001 | 0.884 (0.744–1.050) 0.159 | 0.802 (0.673–0.956) 0.014 | |
| Q3 [263.44–311.77) | N = 2353 | 0.677 (0.584–0.785) < 0.001 | 1.029 (0.798–1.327) 0.826 | 0.864 (0.669–1.116) 0.264 | |
| Q4 (≥ 311.77) | N = 2167 | 0.591 (0.500–0.699) < 0.001 | 1.498 (1.073–2.090) 0.018 | 1.112 (0.780–1.586) 0.556 | |
| P for trend | < 0.001 | 0.153 | 0.865 | ||
| TyG–WC | Continuous | N = 9432 | 1.000 (1.000–1.000) 0.834 | 1.002 (1.001–1.003) < 0.001 | 1.002 (1.001–1.002) < 0.001 |
| Q1 (< 806.88) | N = 2443 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [806.88–914.32) | N = 2467 | 0.880 (0.745–1.039) 0.131 | 0.930 (0.782–1.106) 0.414 | 0.868 (0.730–1.032) 0.109 | |
| Q3 [914.32–1038.15) | N = 2369 | 0.870 (0.740–1.022) 0.090 | 1.063 (0.851–1.329) 0.591 | 0.939 (0.751–1.174) 0.582 | |
| Q4 (≥ 1038.15) | N = 2153 | 0.993 (0.827–1.193) 0.941 | 1.785 (1.338–2.382) < 0.001 | 1.435 (1.055–1.952) 0.022 | |
| P for trend | 0.853 | < 0.001 | 0.052 | ||
| TyG–WHtR | Continuous | N = 9432 | 1.072 (1.009–1.140) 0.025 | 1.525 (1.356–1.715) < 0.001 | 1.417 (1.250–1.608) < 0.001 |
| Q1 (< 4.80) | N = 2274 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [4.80–5.44) | N = 2368 | 0.955 (0.816–1.116) 0.560 | 0.973 (0.841–1.125) 0.709 | 0.935 (0.804–1.086) 0.376 | |
| Q3 [5.44–6.16) | N = 2466 | 1.092 (0.912–1.307) 0.337 | 1.249 (0.997–1.566) 0.053 | 1.142 (0.908–1.435) 0.257 | |
| Q4 (≥ 6.16) | N = 2324 | 1.156 (0.977–1.369) 0.092 | 2.041 (1.597–2.608) < 0.001 | 1.677 (1.288–2.184) < 0.001 | |
| P for trend | 0.045 | < 0.001 | < 0.001 | ||
| Cardiovascular mortality | |||||
| TyG index | Continuous | N = 9432 | 1.259 (1.088–1.457) 0.002 | 1.336 (1.128–1.582) < 0.001 | 1.207 (1.005–1.449) 0.044 |
| Q1 (< 8.39) | N = 2461 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [8.39–8.79) | N = 2338 | 1.268 (0.972–1.656) 0.081 | 1.104 (0.855–1.427) 0.447 | 1.059 (0.820–1.368) 0.661 | |
| Q3 [8.79–9.23) | N = 2310 | 1.218 (0.949–1.565) 0.122 | 1.138 (0.892–1.453) 0.298 | 1.031 (0.811–1.310) 0.804 | |
| Q4 (≥ 9.23) | N = 2323 | 1.514 (1.150–1.993) 0.003 | 1.501 (1.127–1.998) 0.005 | 1.254 (0.930–1.691) 0.137 | |
| P for trend | 0.007 | 0.006 | 0.168 | ||
| TyG–BMI | Continuous | N = 9432 | 0.998 (0.997–1.000) 0.028 | 1.010 (1.005–1.016) < 0.001 | 1.007 (1.001–1.013) 0.016 |
| Q1 (< 223.94) | N = 2431 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [223.94–263.44) | N = 2481 | 0.740 (0.589–0.931) 0.010 | 0.860 (0.631–1.172) 0.339 | 0.775 (0.560–1.073) 0.125 | |
| Q3 [263.44–311.77) | N = 2353 | 0.730 (0.579–0.920) 0.008 | 1.139 (0.742–1.749) 0.552 | 0.945 (0.604–1.479) 0.805 | |
| Q4 (≥ 311.77) | N = 2167 | 0.717 (0.551–0.934) 0.014 | 1.921 (0.995–3.709) 0.052 | 1.401 (0.700–2.803) 0.341 | |
| P for trend | 0.012 | 0.133 | 0.588 | ||
| TyG–WC | Continuous | N = 9432 | 1.000 (1.000–1.001) 0.176 | 1.002 (1.001–1.003) < 0.001 | 1.002 (1.000–1.003) 0.011 |
| Q1 (< 806.88) | N = 2443 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [806.88–914.32) | N = 2467 | 0.894 (0.707–1.132) 0.353 | 0.866 (0.662–1.132) 0.292 | 0.799 (0.608–1.051) 0.109 | |
| Q3 [914.32–1038.15) | N = 2369 | 0.871 (0.678–1.120) 0.282 | 0.958 (0.670–1.371) 0.815 | 0.834 (0.568–1.224) 0.354 | |
| Q4 (≥ 1038.15) | N = 2153 | 1.157 (0.889–1.506) 0.279 | 1.783 (1.159–2.742) 0.009 | 1.404 (0.865–2.278) 0.170 | |
| P for trend | 0.359 | 0.021 | 0.286 | ||
| TyG–WHtR | Continuous | N = 9432 | 1.148 (1.045–1.261) 0.004 | 1.609 (1.351–1.917) < 0.001 | 1.481 (1.217–1.802) < 0.001 |
| Q1 (< 4.80) | N = 2274 | 1(Ref) | 1(Ref) | 1(Ref) | |
| Q2 [4.80–5.44) | N = 2368 | 1.008 (0.768–1.322) 0.955 | 0.965 (0.727–1.281) 0.804 | 0.917 (0.691–1.218) 0.551 | |
| Q3 [5.44–6.16) | N = 2466 | 1.252 (0.964–1.626) 0.092 | 1.340 (0.951–1.888) 0.094 | 1.210 (0.845–1.732) 0.298 | |
| Q4 (≥ 6.16) | N = 2324 | 1.392 (1.055–1.835) 0.019 | 2.257 (1.435–3.550) < 0.001 | 1.812 (1.114–2.948) 0.017 | |
| P for trend | 0.005 | < 0.001 | 0.017 | ||
Bold values indicate statistical significance. A P value < 0.05 was considered to indicate statistical significance
BMI Body mass index; CI Confidence interval; DM Diabetes mellitus; eGFR Estimated glomerular filtration rate; HR Hazard ratio; HTN Hypertension; TC Total cholesterol; TyG Triglyceride–glucose; WC Waist circumference; WHtR Waist-to-height ratio
Model 1: No covariates were adjusted for
Model 2: Adjusted for age, sex, race, smoking status, alcohol consumption status, and BMI
Model 3: Adjusted for age, sex, race, smoking status, alcohol consumption status, BMI, stroke status, DM status, TC levels, and the eGFR
The Cox regression models also revealed significant associations between the TyG index, TyG–BMI, TyG–WC, and TyG–WHtR and cardiovascular mortality, both before and after covariate adjustment (Table 2). Notably, TyG–WHtR had the strongest association, with each 1-unit increase corresponding to a 48.1% increase in cardiovascular mortality risk (P < 0.001).
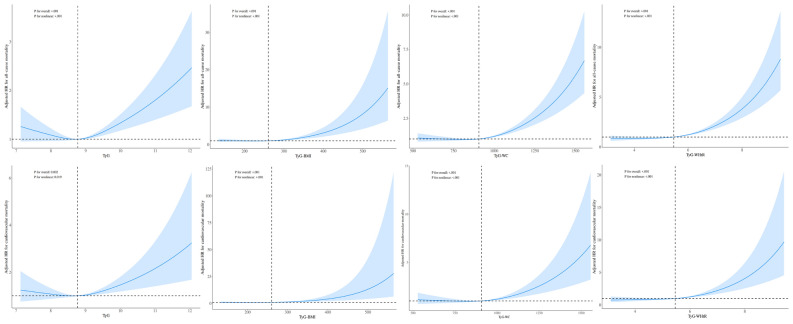
Nonlinear relationships between the TyG index and mortality
A multivariable Cox proportional hazards regression model with RCS analysis was used to assess the nonlinear relationships of the TyG index and its derived indices with all-cause mortality and cardiovascular mortality. After adjusting for covariates, a significant L-shaped relationship was observed for the TyG index and its derived indices with both all-cause (nonlinear P < 0.001) and cardiovascular mortality (nonlinear P < 0.05), as depicted in Fig. 2.
Fig. 2.
Associations of the TyG index and its derivatives with all-cause and cardiovascular mortality in patients with HTN. BMI, Body mass index; HR, Hazard ratio; HTN, Hypertension; TyG, Triglyceride–glucose; WC, Waist circumference; WHtR, Waist-to-height ratio
Feature selection in machine learning
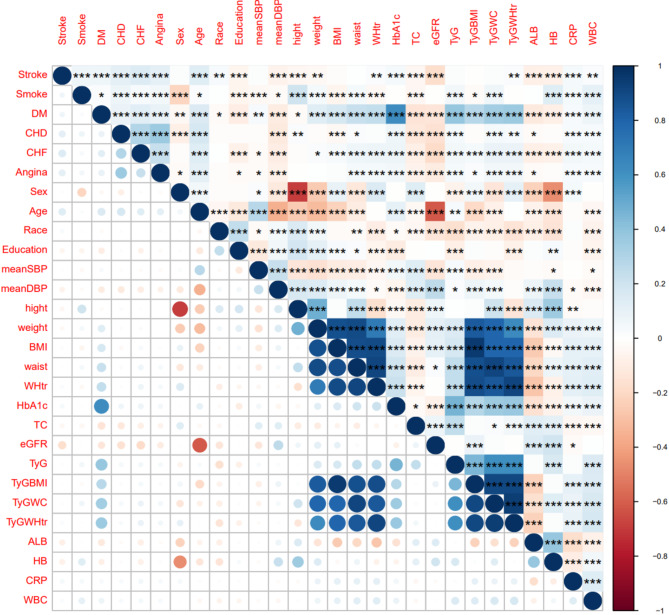
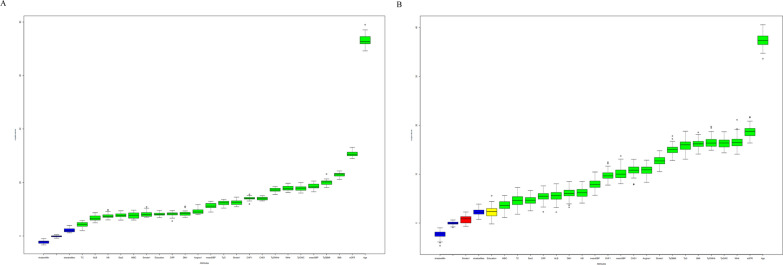
The correlation matrix for the study variables is presented in Fig. 3, and the significant relationships are highlighted. Age was significantly negatively correlated with the eGFR, and there was a significant negative correlation between height and sex. Additionally, the TyG index and its combined obesity indices were significantly positively correlated with height, weight, BMI, WC and WHtR. Feature selection using the Boruta algorithm identified the TyG index and its associated indices as significant predictors of adverse outcomes in hypertensive patients (Fig. 4). LASSO regression further refined the selection. While 10 variables were ultimately selected for predicting all-cause mortality, only 3 variables (age, angina, and mean SBP) were chosen for cardiovascular mortality. However, the limited number of variables for cardiovascular mortality led to poor predictive performance (AUROC = 0.548). To address this, we included the same variables used for all-cause mortality, aiming to improve the model’s predictive ability and to facilitate a better assessment of the incremental predictive value of TyG and its derivatives across different outcomes. The final variables included in the basic model were age, BMI, CHF status, CRP levels, the eGFR, education level, mean SBP, smoking status, TC levels, and the WBC count. The detailed results of the feature selection processes are provided in Supplementary Table 6 and Supplementary Fig. 2. On the basis of the basic model, we constructed four improved models by incorporating the TyG index and its derivatives separately to assess their predictive value for fatal events.
Fig. 3.
Pearson's correlations among the baseline clinical data. ALB, Albumin; BMI, Body mass index; CHD, Coronary heart disease; CHF, Congestive heart failure; CRP, C-reactive protein; DBP, Diastolic blood pressure; DM, Diabetes mellitus; eGFR, Estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; SBP, Systolic blood pressure; TC, Total cholesterol; TyG, Triglyceride‒glucose; WBC, White blood cell; WC, Waist circumference; WHtR, Waist-to-height ratio
Fig. 4.
Feature selection based on the Boruta algorithm. a All-cause mortality. b Cardiovascular mortality. ALB, Albumin; BMI, Body mass index; CHD, Coronary heart disease; CHF, Congestive heart failure; CRP, C-reactive protein; DBP, Diastolic blood pressure; DM, Diabetes mellitus; eGFR, Estimated glomerular filtration rate; HB, haemoglobin; SBP, Systolic blood pressure; TC, Total cholesterol; TyG, Triglyceride–glucose; WBC, White blood cell; WC, Waist circumference; WHtR, Waist-to-height ratio
Model development and validation
The hyperparameters, tuning ranges, and optimal parameters for the machine learning models are presented in Supplementary Table 1. The performance of the six basic models for all-cause and cardiovascular mortality are compared in Table 3. In the all-cause mortality basic model, the AUROC for logistic regression was 0.8115, the K-NN was 0.8028, the naive Bayes model was 0.8041, the SVM was 0.8133, the random forest model was 0.8227, and XGBoost was 0.8262. Similarly, in the cardiovascular mortality basic model, logistic regression had an AUROC of 0.7612, K-NN had 0.7567, naive Bayes had 0.7618, SVM had 0.6168, random forests had 0.7579, and XGBoost had 0.7687.
Table 3.
The performance of six basic models constructed with all-cause mortality and cardiovascular mortality as the outcome variables
| Basic model | AUC |
|---|---|
| All-cause mortality | |
| Logistic | 0.8114948 |
| K-NN | 0.8028286 |
| Naive Bayes | 0.8041313 |
| SVM | 0.8132667 |
| Random forest | 0.8227461 |
| XGBoost | 0.8261726 |
| Cardiovascular mortality | |
| Logistic | 0.7612384 |
| K-NN | 0.7567034 |
| Naive Bayes | 0.7617524 |
| SVM | 0.616799 |
| Random forest | 0.7579192 |
| XGBoost | 0.7686703 |
K-NN k-nearest neighbours; SVM Support vector machine; XGBoost Extreme gradient boosting
Incremental predictive value of the TyG index and its derivatives
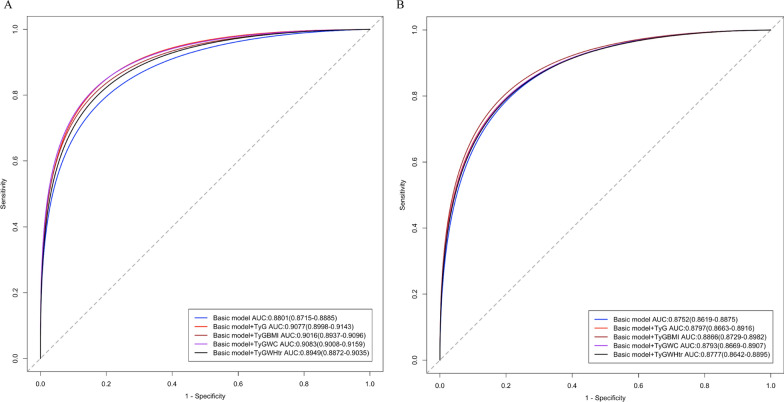
The incremental predictive value of the TyG index, TyG–BMI, TyG–WC, and TyG–WHtR for all-cause and cardiovascular mortality are summarized in Table 4. The addition of these indices to the basic model improved the C statistic. Notably, in the modified all-cause mortality model, both the TyG index and TyG–WC yielded the most substantial improvements. In the cardiovascular model, the TyG–BMI displayed the greatest enhancement, as shown in Fig. 5. Overall, integrating the TyG index and its derivatives enhanced risk stratification and discrimination for mortality outcomes in hypertensive patients.
Table 4.
Incremental predictive value of the cumulative TyG index
| Models | AUC (95% CI) | P value |
|---|---|---|
| All-cause mortality | ||
| Basic model | 0.880 (0.872–0.889) | Ref |
| Basic model + TyG index | 0.908 (0.900–0.914) | < 0.001 |
| Basic model + TyG–BMI | 0.902 (0.894–0.910) | < 0.001 |
| Basic model + TyG–WC | 0.908 (0.901–0.916) | < 0.001 |
| Basic model + TyG–WHtR | 0.895 (0.887–0.904) | 0.013 |
| Cardiovascular mortality | ||
| Basic model | 0.875 (0.862–0.888) | |
| Basic model + TyG index | 0.880 (0.866–0.882) | 0.04 |
| Basic model + TyG–BMI | 0.887 (0.873–0.898) | < 0.001 |
| Basic model + TyG–WC | 0.879 (0.867–0.891) | 0.014 |
| Basic model + TyG–WHtR | 0.878 (0.864–0.890) | 0.072 |
Basic model: Adjusted for age, education level, mean SBP, smoking status, BMI, CHF status, the eGFR, TC levels, and the WBC count. Bold values indicate statistical significance. A P value < 0.05 was considered to indicate statistical significance
AUC Area under the receiver operating characteristic curve; BMI Body mass index; CHF Congestive heart failure; CI Confidence interval; eGFR, Estimated glomerular filtration rate; SBP Systolic blood pressure; TC Total cholesterol; TyG Triglyceride–glucose; WBC White blood cell; WC Waist circumference; WHtR Waist-to-height ratio
Fig. 5.
Efficacy of the basic model and four modified models. a All-cause mortality. b Cardiovascular mortality. AUROC, Area under the receiver operating characteristic curve; TyG, Triglyceride‒glucose; WC, Waist circumference; WHtR, Waist-to-height ratio
Discussion
To our knowledge, this is the first study in which machine learning and multidimensional approaches were used to investigate the associations of the TyG index and its obesity-related derivatives (TyG–BMI, TyG–WC, and TyG–WHtR) with all-cause and cardiovascular mortality in patients with HTN. The key findings of our study are as follows: (1) The TyG index and its derivatives are independent predictors of both all-cause mortality and cardiovascular mortality in hypertensive patients. (2) Among the indices, TyG–WHtR showed the strongest association with mortality outcomes, with a 1-unit increase associated with a 41.7% and 48.1% increased risk of all-cause mortality and cardiovascular mortality, respectively. (3) There was an L-shaped relationship of the TyG index and its derivatives with all-cause and cardiovascular mortality. (4) Incorporating the TyG index and its derivatives into predictive models modestly improved the prediction of mortality outcomes, with the TyG index and TyG–WC providing the greatest improvement in predicting all-cause mortality, whereas the TyG–BMI yielded the most significant increase in cardiovascular mortality prediction.
HTN remains a major global public health issue, and its coexistence with IR poses a dual burden that accelerates cardiovascular and metabolic complications. The TyG index and its derivatives have emerged as reliable surrogate markers of IR, providing critical insights into the link between metabolic dysfunction and adverse cardiovascular outcomes. A study by Tao et al. [19] reported that an elevated TyG index is a significant prognostic marker for adverse events in patients with both CHD and HTN, with a 47.0% increased risk of major adverse cardiovascular events (MACEs) in the highest TyG index group over a 1-year follow-up [odds ratio (OR): 1.470, 95% CI 1.071–2.018]. Similarly, a post hoc analysis from the Systolic Blood Pressure Intervention Trial (SPRINT) revealed that a 1-unit increase in the TyG index was associated with a greater risk of MACEs (HR: 1.40, 95% CI 1.20–1.64, P < 0.01), with no significant sex-based differences (P = 0.0776) [20]. In patients with chronic coronary syndrome undergoing percutaneous coronary intervention, the TyG index also demonstrated predictive utility for MACE occurrence [21]. Further studies have combined obesity-related metrics with the TyG index to derive novel indices with improved predictive capabilities. Dang et al. [22] reported that the TyG–WHtR was the strongest predictor of cardiovascular mortality (HR: 1.66, 95%: CI 1.21–2.29), outperforming the TyG index and TyG–WC in terms of diagnostic accuracy. Additionally, Li et al. [23] demonstrated a significant association between the TyG–BMI and CVD incidence in a Chinese population (OR: 1.168, 95% CI 1.040–1.310). The growing global prevalence of HTN and IR, as highlighted in these studies, aligns with our findings and reinforces the consistent epidemiological evidence supporting the TyG index as a robust risk stratification tool.
Our analysis confirmed that the TyG index and its derivatives are strongly associated with both all-cause and cardiovascular mortality, maintaining their predictive value even after multivariable adjustment. Although some HRs in our study appeared to be moderate, they remained statistically significant and clinically meaningful, particularly given the long follow-up period and the high-risk profile of the hypertensive population. For example, a 13.1% increase in all-cause mortality risk per 1-unit increase in the TyG index was substantial over a nearly 10-year mean follow-up. Moreover, the 41.7% and 48.1% elevated risks associated with a 1-unit increase in the TyG–WHtR for all-cause and cardiovascular mortality, respectively, represent significant increases in risk that warrant clinical attention. Our analysis confirmed that the TyG index and its derivatives remain significant predictors of mortality even after adjusting for multiple confounders, underscoring their utility in risk stratification among hypertensive patients. Despite the moderate magnitude of some associations, the consistent significance across models and indices highlights the robustness of these predictors. In the context of complex, multifactorial diseases such as HTN, even modest associations can have substantial clinical implications, particularly when they help identify individuals at higher risk who may benefit from targeted interventions.
Consistent with prior research linking IR, metabolic dysfunction, and cardiovascular outcomes [24–27], our findings underscore the utility of these indices in cardiovascular risk assessment. Among the derived indices, the TyG–WHtR demonstrated the strongest correlation with mortality outcomes, highlighting its potential for use in clinical risk stratification. The L-shaped associations between TyG-related indices and mortality observed in the RCS analysis further validated the increased risks associated with elevated indices. Discrepancies with previous studies should be noted. Hou et al. [28] reported no significant associations between the TyG index or TyG–BMI and survival outcomes in patients with CHD and HTN. In contrast, our results indicate that the TyG index and TyG–WC provide the greatest improvement in predicting all-cause mortality, whereas the TyG–BMI offers superior predictive performance for cardiovascular mortality. These differences may stem from variations in study populations, racial diversity, follow-up durations, or differences in BMI thresholds and definitions of metabolic obesity across populations.
Machine learning offers distinct advantages in handling complex datasets and capturing nonlinear relationships between variables. Unlike traditional statistical methods, which often assume linear associations, machine learning models excel in identifying intricate patterns and interactions among metabolic indices, sociodemographic factors, and mortality outcomes. In our study, XGBoost outperformed the other models, demonstrating superior predictive accuracy. By integrating machine learning models into clinical decision support systems, clinicians may achieve more precise risk stratification and tailor interventions to high-risk hypertensive patients [29]. The incorporation of TyG-related indices into a machine learning framework facilitates the early identification of hypertensive patients at the highest risk for mortality, enabling timely and targeted interventions. This approach aligns with the principles of precision medicine, with the potential to optimize resource allocation and improve patient outcomes.
There are potential interactive mechanisms linking HTN, IR, and other CVDs. IR exacerbates endothelial dysfunction, promotes vascular inflammation, and accelerates atherosclerosis, which are key contributors to CVD [30, 31]. Similarly, HTN induces haemodynamic stress, leading to left ventricular hypertrophy and vascular remodelling [32, 33]. The TyG index, as a surrogate marker for IR, captures these metabolic disturbances. When combined with obesity-related metrics such as WC or WHtR, this index offers a comprehensive assessment of cardiometabolic risk by reflecting both central obesity and its metabolic consequences.
Our study has several limitations that warrant acknowledgement. First, the observational design of the NHANES data restricts causal inference. Although we adjusted for multiple confounders, residual confounding cannot be entirely ruled out. Second, reliance on self-reported data for certain variables, such as smoking and alcohol consumption, may introduce reporting bias. Third, the study cohort primarily comprised individuals from the United States, which may limit the generalizability of our findings to populations with different genetic, environmental, and lifestyle factors. Finally, while the machine learning models demonstrated high predictive accuracy, they require external validation in diverse cohorts to ensure robustness and applicability in clinical settings.
Conclusions
In conclusion, our study highlights the TyG index and its derivatives as independent predictors of both all-cause and cardiovascular mortality in hypertensive patients. Incorporating TyG-related indices into a machine learning framework enhances the early identification of hypertensive patients at the highest risk of adverse outcomes, enabling timely and precise interventions. Machine learning techniques offer potential advantages in improving individualized risk assessments. Future research should focus on validating these findings in other hypertensive cohorts and integrating these indices into clinical practice to enhance the early detection and management of high-risk hypertensive patients.
Supplementary Information
Acknowledgements
The authors thank the NHANES database for providing the data source for this study. The authors thank all the study participants for their contribution to the research.
Abbreviations
- ALB
Albumin
- AUROC
Area under the receiver operating characteristic curve
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- CHD
Coronary heart disease
- CHF
Congestive heart failure
- CI
Confidence interval
- CRP
C-reactive protein
- C-statistic
Concordance statistic
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- FPG
Fasting plasma glucose
- Hb
Haemoglobin
- HbA1c
Glycosylated haemoglobin
- HR
Hazard ratio
- HTN
Hypertension
- IR
Insulin resistance
- K–M
Kaplan–Meier
- K-NN
K-nearest neighbours
- LASSO
Least absolute shrinkage and selection operator
- MACE
Major adverse cardiovascular event
- NCHS
National Center for Health Statistics
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
- RCS
Restricted cubic spline
- SBP
Systolic blood pressure
- SVM
Support vector machines
- TC
Total cholesterol
- TG
Triglyceride
- TyG
Triglyceride–glucose
- WBC
White blood cell
- WC
Waist circumference
- WHtR
Waist-to-height ratio
- XGBoost
Extreme gradient boosting
Author contributions
Yongguo Dai and Zeying Zhang collected the relevant information; Cancan Wang, Yichao Xiao, and Xiaoqin Luo performed the statistical analysis; Tao Tu and Chan Liu performed the data visualization; Chenyang Li and Zixi Zhang wrote the manuscript, which was subsequently revised by Qiming Liu, Zheng Cheng, and Jiafeng Lin. All the authors contributed to the discussion and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [Nos. 82070356, 81770337, 82470333, 82300357, 82070333], the Chinese Society of Cardiology’s Foundation [No. CSCF2024B02], the Hunan Provincial Natural Science Foundation of China [Nos. 2021JJ30033, 2023JJ30791], the Key Project of Hunan Provincial Science and Technology Innovation [No. 2024JK2119], the Wenzhou Municipal Science and Technology Commission [No. Y20220472], and the Clinical Medical Technology Innovation Guidance Project of Hunan Science and Technology Agency [No. 2021SK53519].
Data availability
The NHANES data were collected by the NCHS, a division of the CDC of the United States. The data are released for research purposes and can be accessed with permission from the NCHS at https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
The NHANES is a survey of the nutritional and health status of a nationally representative population in the United States. conducted by the NCHS. The survey protocol was approved by the NCHS ethics review board, and all participants provided signed informed consent. All the authors participated in the study and made significant intellectual contributions to the manuscript.
Consent for publication
This manuscript is not currently under consideration for publication elsewhere, and the work reported will not be submitted for publication elsewhere until a final decision has been made as to its acceptability by the journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenyang Li and Zixi Zhang contributed equally to this work.
Contributor Information
Cheng Zheng, Email: zhengcheng_wzmufey@163.com.
Jiafeng Lin, Email: linjiafeng_wzmcfey@163.com.
References
- 1.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- 3.Gazit T, Gutman M, Beatty AL. Assessment of hypertension control among adults participating in a mobile technology blood pressure self-management program. JAMA Netw Open. 2021;4: e2127008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Zhou X, Zhuo X, Zhang P. Annual total medical expenditures associated with hypertension by diabetes status in U.S adults. Am J Prev Med. 2017;53:S182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver CG, Clement FM, Campbell NR, James MT, Klarenbach SW, Hemmelgarn BR, et al. Healthcare costs attributable to hypertension: Canadian population-based cohort study. Hypertension. 2015;66:502–8. [DOI] [PubMed] [Google Scholar]
- 6.Kirkland EB, Heincelman M, Bishu KG, Schumann SO, Schreiner A, Axon RN, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc. 2018;7: e008731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zick Y. Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 2001;11:437–41. [DOI] [PubMed] [Google Scholar]
- 8.Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the veterans affairs HDL intervention trial (VA-HIT). Diabetes Care. 2003;26:1513–7. [DOI] [PubMed] [Google Scholar]
- 9.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–75. [DOI] [PubMed] [Google Scholar]
- 10.Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108:2074–81. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Xu W, Song T, Wang X, Wang Q, Li J, et al. Changes in the combination of the triglyceride-glucose index and obesity indicators estimate the risk of cardiovascular disease. Cardiovasc Diabetol. 2024;23:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Q, Huang Y, Liu Q, Chu T, Li G, Wu Z. Association between triglyceride glucose-waist height ratio index and cardiovascular disease in middle-aged and older Chinese individuals: a nationwide cohort study. Cardiovasc Diabetol. 2024;23:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang F, Tang J, Shen L, He J, Chen Y. Association of triglyceride glucose-related parameters with all-cause mortality and cardiovascular disease in NAFLD patients: NHANES 1999–2018. Cardiovasc Diabetol. 2024;23:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. [DOI] [PubMed] [Google Scholar]
- 16.Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride glucose index and related parameters (Triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19:167–73. [DOI] [PubMed] [Google Scholar]
- 17.Malek M, Khamseh ME, Chehrehgosha H, Nobarani S, Alaei-Shahmiri F. Triglyceride glucose-waist to height ratio: a novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine. 2021;74:538–45. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao S, Yu L, Li J, Huang L, Huang X, Zhang W, et al. Association between the triglyceride-glucose index and 1-year major adverse cardiovascular events in patients with coronary heart disease and hypertension. Cardiovasc Diabetol. 2023;22:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Liu W. Triglyceride and glucose index and sex differences in relation to major adverse cardiovascular events in hypertensive patients without diabetes. Front Endocrinol (Lausanne). 2021;12: 761397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao S, Yu L, Li J, Xie Z, Huang L, Yang D, et al. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2023;22:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Wang Y, Shi B, Sun S, Wang S, Pang S, et al. Association between the cumulative average triglyceride glucose-body mass index and cardiovascular disease incidence among the middle-aged and older population: a prospective nationwide cohort study in China. Cardiovasc Diabetol. 2024;23:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He J, Song C, Yuan S, Bian X, Lin Z, Yang M, et al. Triglyceride-glucose index as a suitable non-insulin-based insulin resistance marker to predict cardiovascular events in patients undergoing complex coronary artery intervention: a large-scale cohort study. Cardiovasc Diabetol. 2024;23:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Shen C, Kong W, Zhou X, Fan H, Zhang Y, et al. Association between the triglyceride glucose-body mass index and future cardiovascular disease risk in a population with Cardiovascular-Kidney-Metabolic syndrome stage 0–3: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui C, Qi Y, Song J, Shang X, Han T, Han N, et al. Comparison of triglyceride glucose index and modified triglyceride glucose indices in prediction of cardiovascular diseases in middle aged and older Chinese adults. Cardiovasc Diabetol. 2024;23:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X, Min Y, Song G, Ye X, Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. 2024;23:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou XZ, Lv YF, Li YS, Wu Q, Lv QY, Yang YT, et al. Association between different insulin resistance surrogates and all-cause mortality in patients with coronary heart disease and hypertension: NHANES longitudinal cohort study. Cardiovasc Diabetol. 2024;23:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Heuvel L, Dorsey RR, Prainsack B, Post B, Stiggelbout AM, Meinders MJ, et al. Quadruple decision making for Parkinson’s disease patients: combining expert opinion, patient preferences, scientific evidence, and big data approaches to reach precision medicine. J Parkinsons Dis. 2020;10:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feijoo-Bandin S, Aragon-Herrera A, Morana-Fernandez S, Anido-Varela L, Tarazon E, Rosello-Lleti E, et al. Adipokines and inflammation: focus on cardiovascular diseases. Int J Mol Sci. 2020;21:7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohlmorgen C, Gerfer S, Feldmann K, Twarock S, Hartwig S, Lehr S, et al. Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia. 2021;64:1834–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Li W, Li L, Yang S, Zhao G, Li K. Association between blood groups and myocardial injury after non-cardiac surgery: a retrospective cohort study. Sci Rep. 2024;14:14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masenga SK, Kirabo A. Hypertensive heart disease: risk factors, complications and mechanisms. Front Cardiovasc Med. 2023;10:1205475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES data were collected by the NCHS, a division of the CDC of the United States. The data are released for research purposes and can be accessed with permission from the NCHS at https://www.cdc.gov/nchs/nhanes/index.htm.