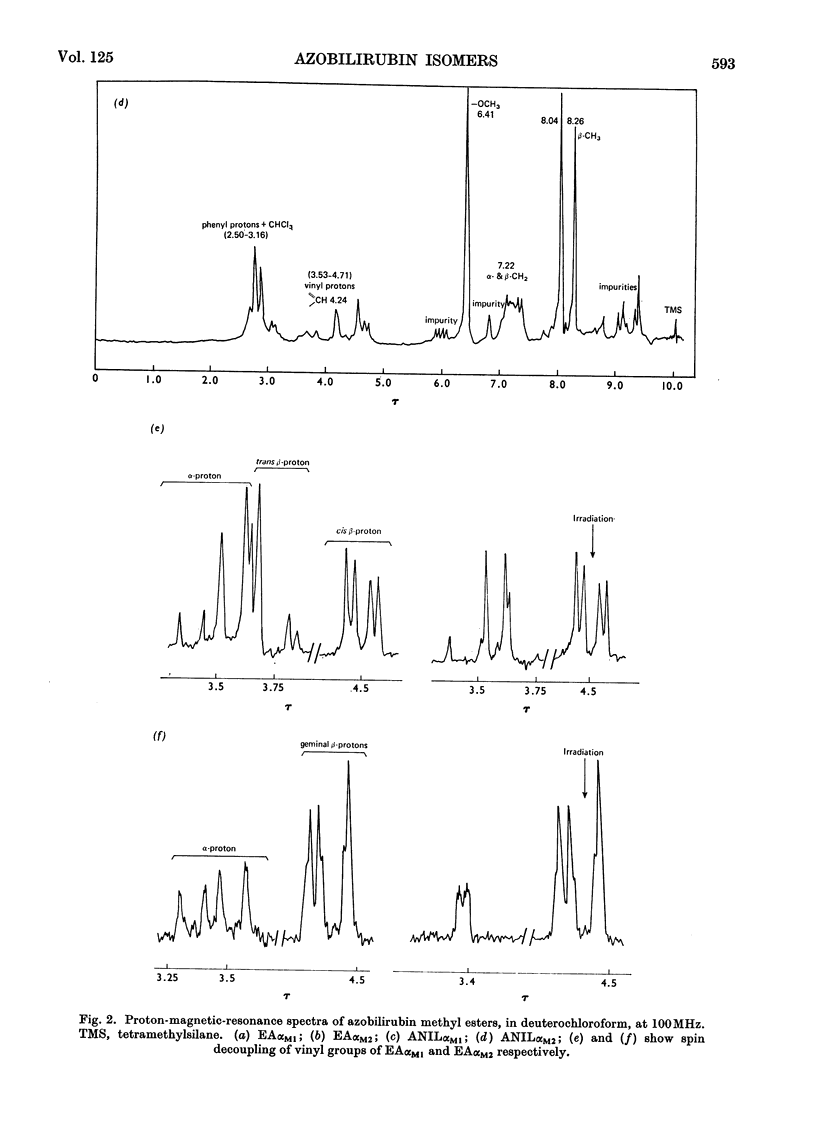
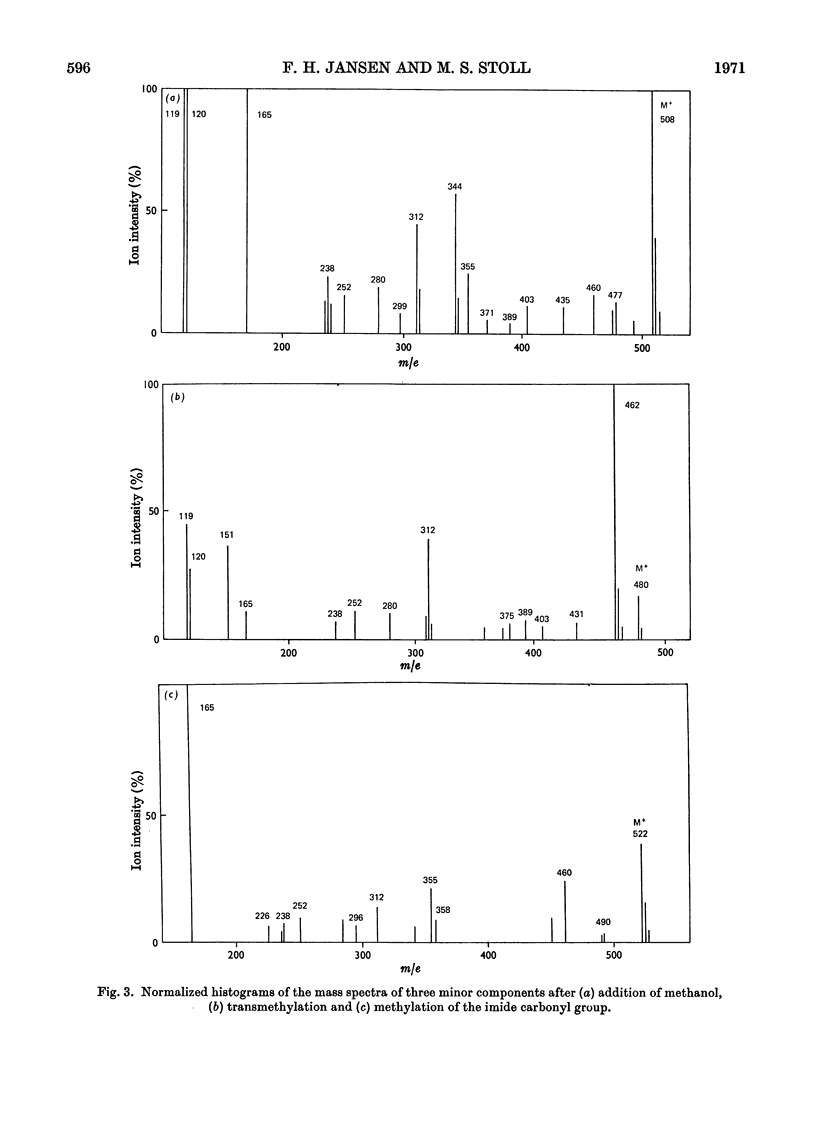
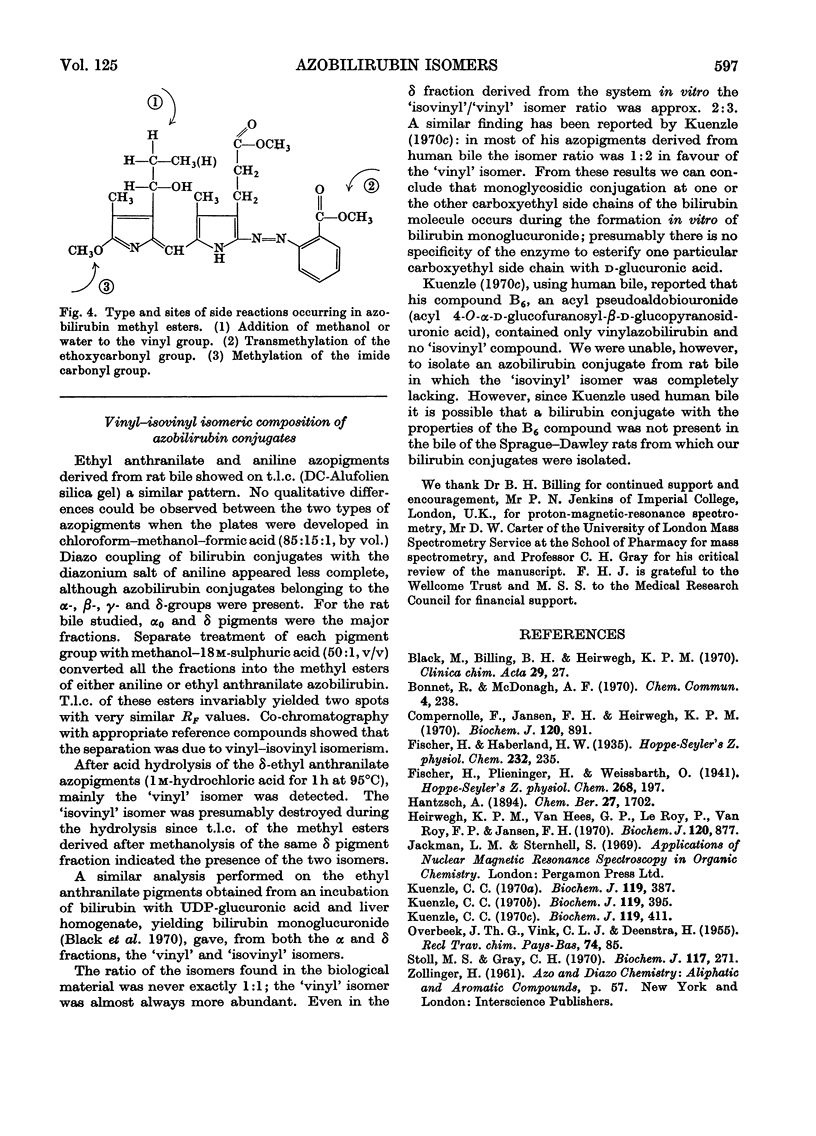
Abstract
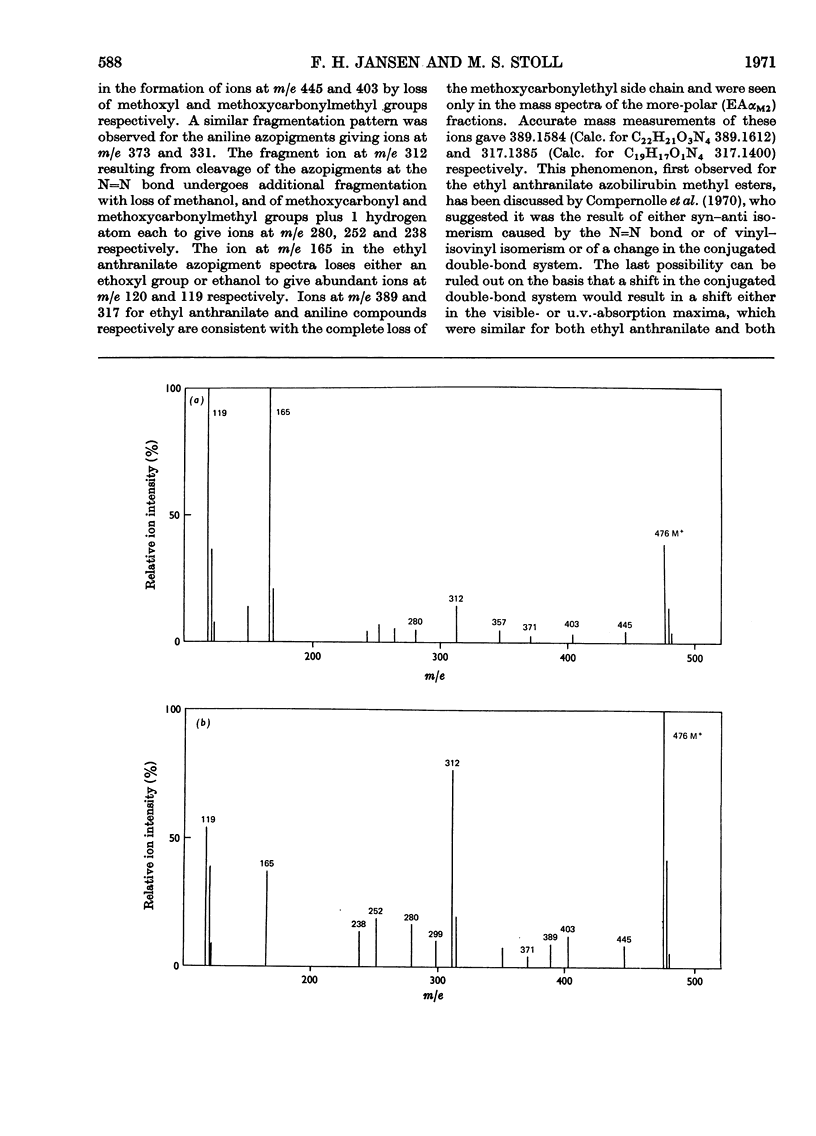
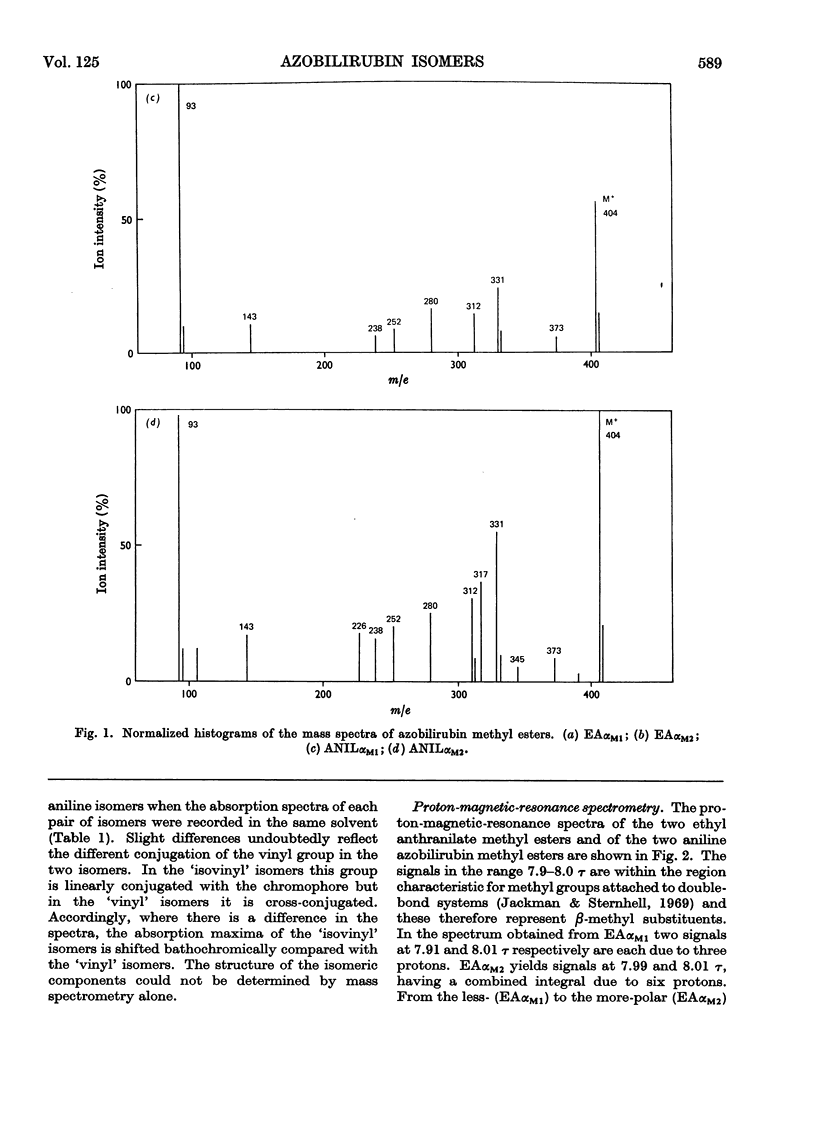
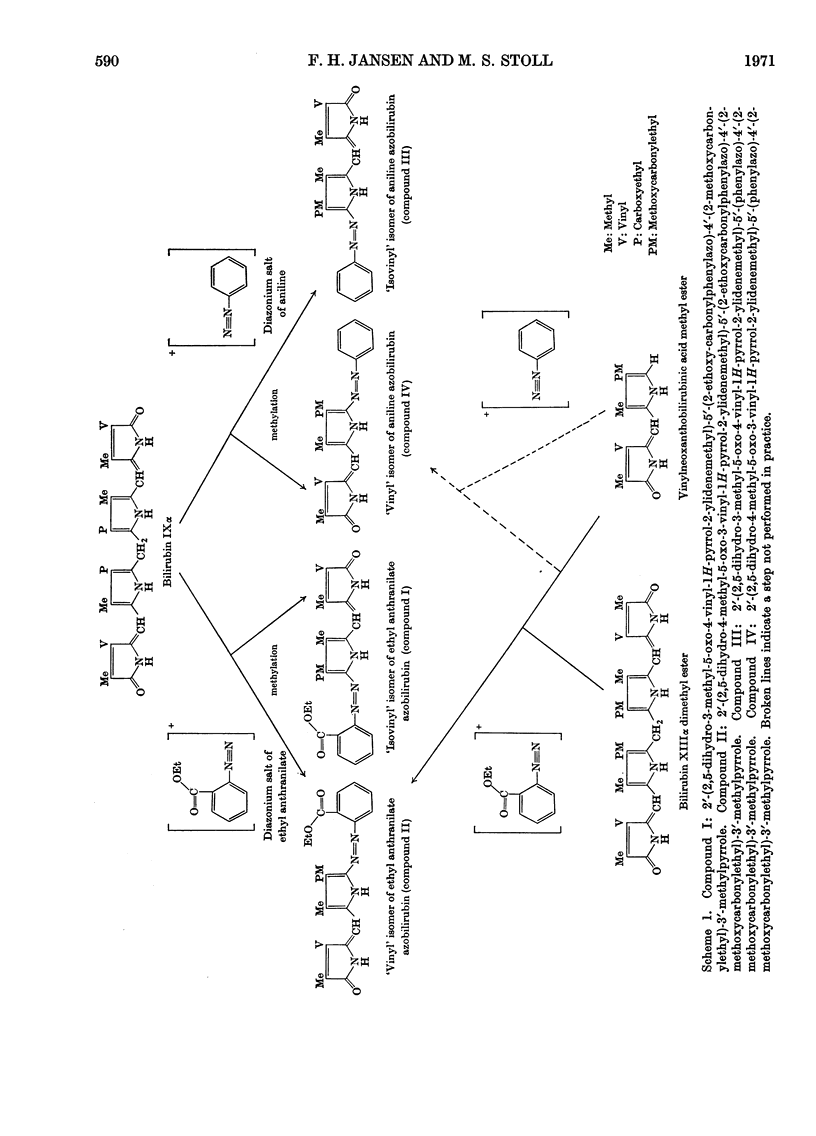
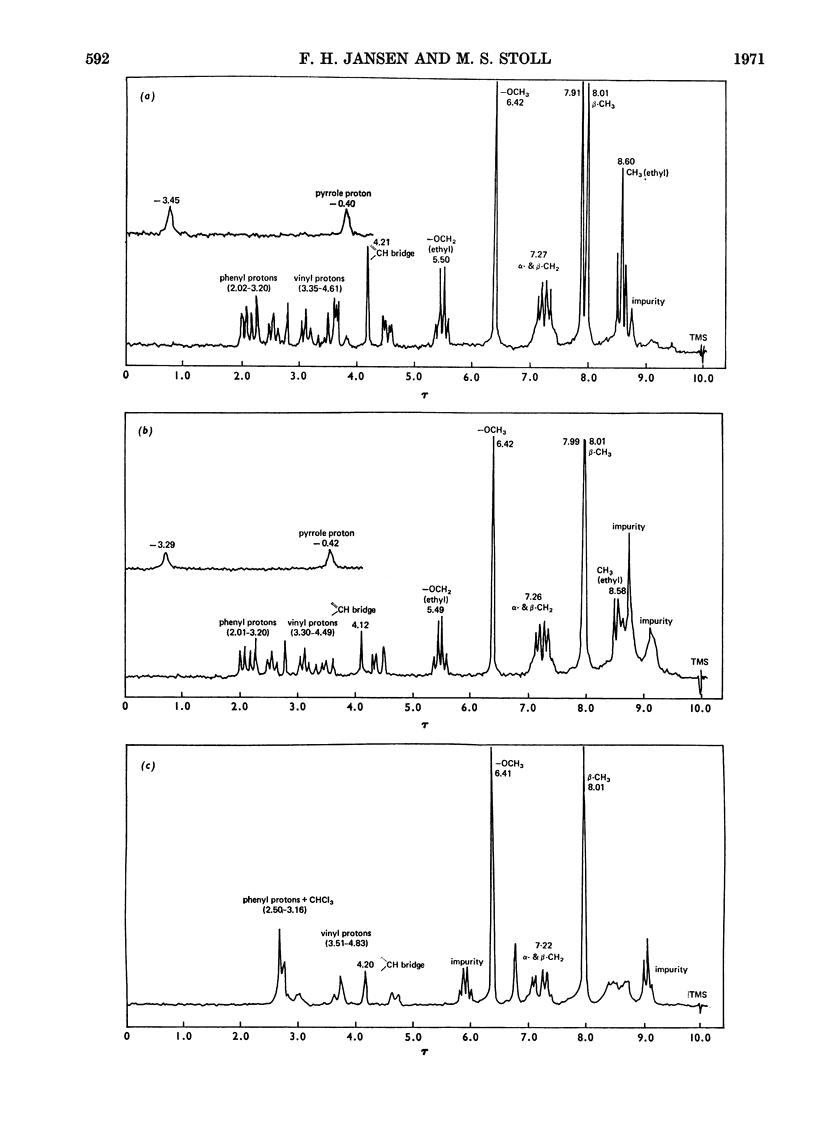
The coupling reaction of bilirubin with the diazonium salts of ethyl anthranilate or of aniline yields two isomeric azopigments. These can be separated by t.l.c. as their methyl esters. The mass spectra of each pair of azopigments are very similar, showing that they are isomers. Proton-magnetic-resonance spectrometric studies show that they differ in the positions of the substituents on the pyrrolenone end ring; in one compound the methyl and vinyl groups are interposed compared with the other compound. These azo compounds were used as reference standards for determination of the site of conjugation in bilirubin monoglucuronide prepared enzymically. Analysis showed that conjugation occurs at the carboxyethyl side chain of both sides of the bilirubin molecule. During the preparation of the ethyl anthranilate reference compounds a series of minor azopigments were isolated by t.l.c. Analysis of the mass spectra of many of these showed that three side reactions can occur: (1) methylation of the imide carbonyl group; (2) addition of methanol or water to the vinyl substituent; (3) transmethylation of the ethoxycarbonyl group.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black M., Billing B. H., Heirwegh K. P. Determination of bilirubin UDP-glucuronyl transferase activity in needle-biopsy specimens of human liver. Clin Chim Acta. 1970 Jul;29(1):27–35. doi: 10.1016/0009-8981(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Isolation of phenylazo derivatives of bile bilirubin. Biochem J. 1970 Sep;119(3):387–394. doi: 10.1042/bj1190387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll M. S., Gray C. H. The oxidation products of crude mesobilirubinogen. Biochem J. 1970 Apr;117(2):271–290. doi: 10.1042/bj1170271. [DOI] [PMC free article] [PubMed] [Google Scholar]


