Abstract
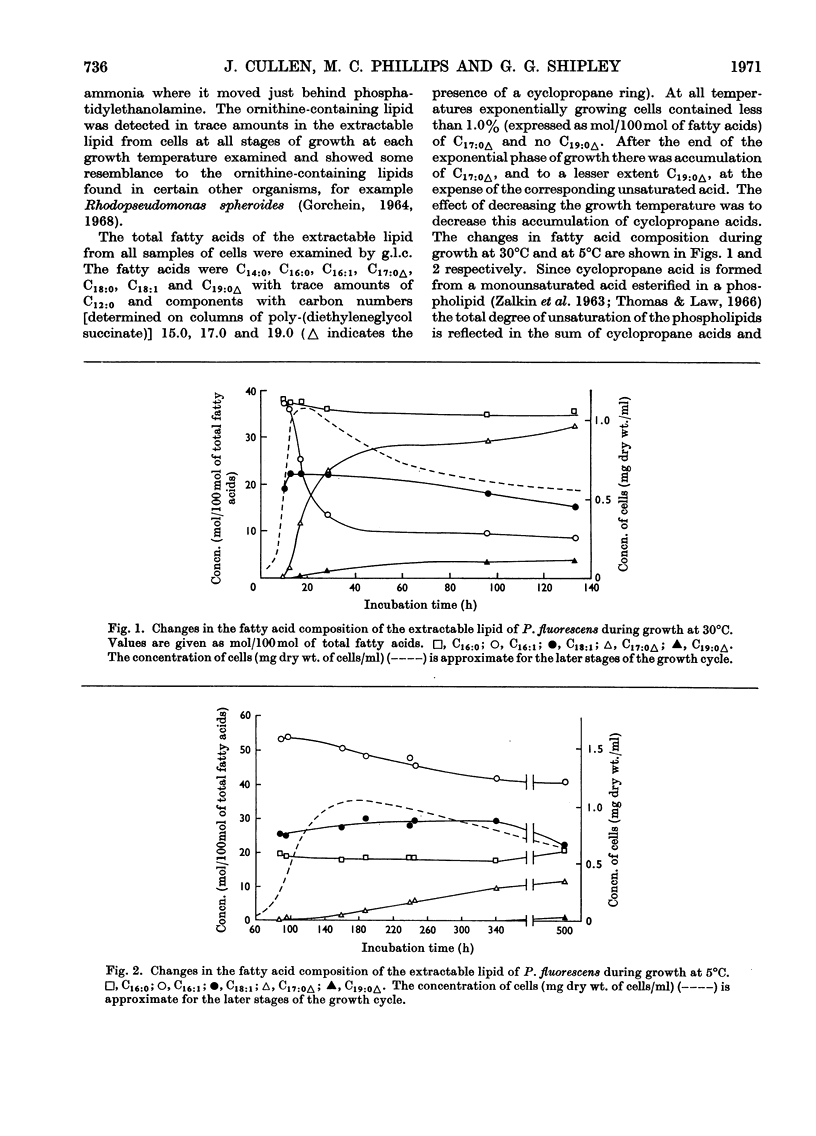
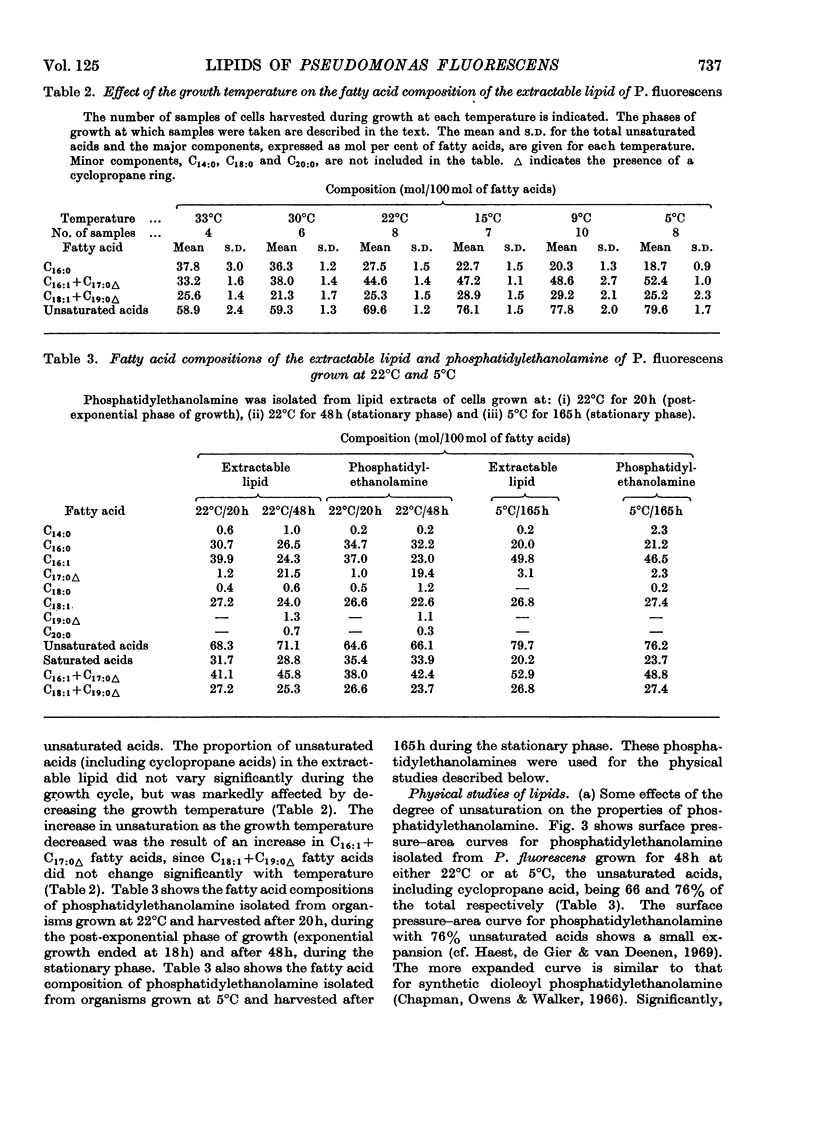
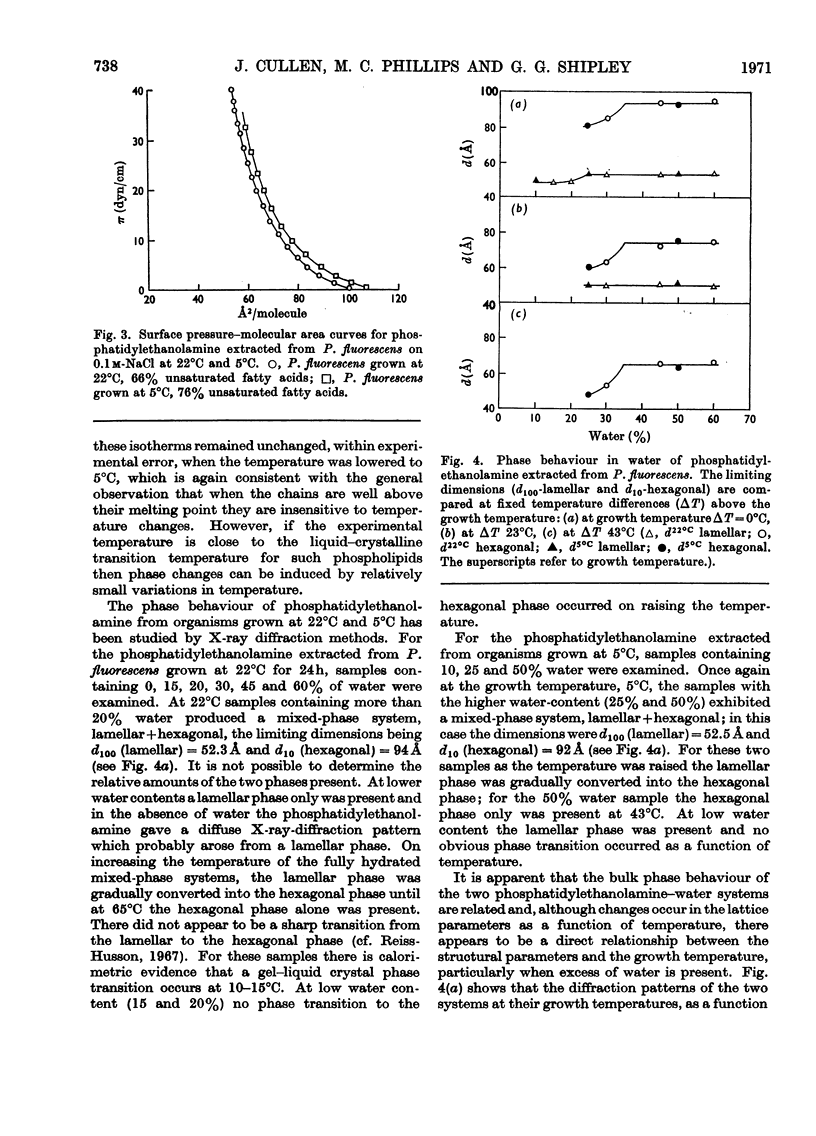
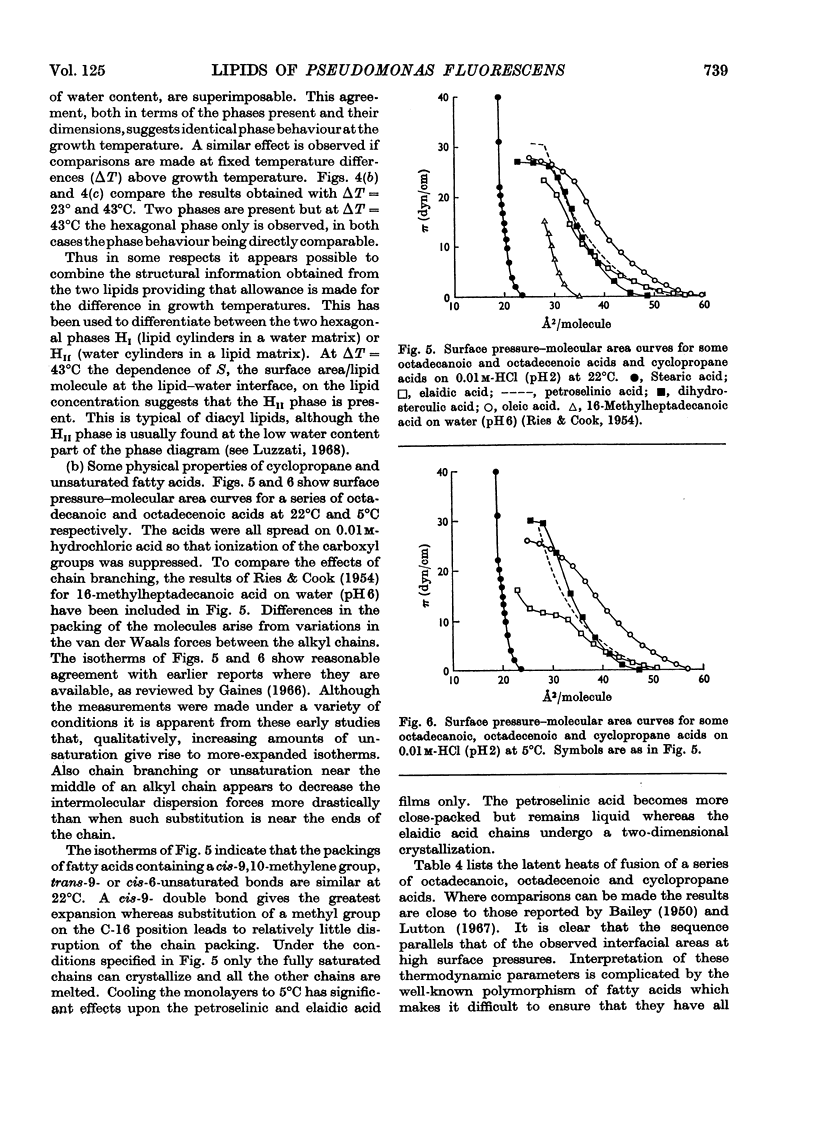
1. Pseudomonas fluorescens was grown at various temperatures between 5°C and 33°C. The extractable lipids from organisms at various stages of growth and grown at different temperatures were examined. 2. The extractable lipids contained phosphatidylethanolamine, diphosphatidylglycerol, phosphatidylglycerol, phosphatidylcholine, and an ornithine-containing lipid. The relative amounts of these lipids did not vary significantly during growth or with the changes in growth temperature. 3. The major fatty acids were hexadecanoic, hexadecenoic and octadecenoic acids and the cyclopropane acids methylene-hexadecanoic and methylene-octadecanoic acids. The relative amount of unsaturated acids (including cyclopropane acids) did not change significantly during growth, but increased with decreasing temperature. 4. Phosphatidylethanolamines with different degrees of unsaturation and containing different amounts of cyclopropane acids were isolated from organisms grown at 5°C and 22°C and their surface and phase behaviour in water was investigated. Thermodynamic parameters for fusion and monolayer results for cyclopropane and other fatty acids were examined. 5. The surface pressure–area isotherms of phosphatidylethanolamines containing different amounts of unsaturated fatty acids show small differences but the individual isotherms remain essentially unchanged over the temperature range 5–22°C. X-ray-diffraction methods show that the structures (lamellar+hexagonal) formed in water by phosphatidylethanolamine, isolated from organisms grown at 5°C and 22°C, are identical when compared at the respective growth temperatures. This points to a control mechanism of the physical state of the lipids that is sensitive to the operating temperature of the organism. 6. The molecular packing of cyclopropane acids is intermediate between that of the corresponding cis- and trans-monoenoic acids. However, substitution of a cyclopropane acid for a cis-unsaturated acid has insignificant effects on the molecular packing of phospholipids containing these acids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLOU C. E., VILKAS E., LEDERER E. Structural studies on the myo-inositol phospholipids of Mycobacterium tuberculosis (var. bovis, strain BCG). J Biol Chem. 1963 Jan;238:69–76. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BENSON A. A., STRICKLAND E. H. Plant phospholipids. 3. Identification of diphosphatidyl glycerol. Biochim Biophys Acta. 1960 Jul 1;41:328–333. doi: 10.1016/0006-3002(60)90016-0. [DOI] [PubMed] [Google Scholar]
- Birge C. H., Silbert D. F., Vagelos P. R. A beta-hydroxydecanoyl-ACP dehydrase specific for saturated fatty acid biosynthesis in E. coli. Biochem Biophys Res Commun. 1967 Dec 29;29(6):808–814. doi: 10.1016/0006-291x(67)90291-4. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL K. K. Quantitative estimation of peak areas in gas-liquid chromatography. Nature. 1961 Jul 22;191:377–378. doi: 10.1038/191377a0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Owens N. F., Walker D. A. Physical studies of phospholipids. II. Monolayer studies of some synthetic 2,3-diacyl-DL-phosphatidylethanolamines and phosphatidylcholines containing trans double bonds. Biochim Biophys Acta. 1966 May 12;120(1):148–155. doi: 10.1016/0926-6585(66)90286-x. [DOI] [PubMed] [Google Scholar]
- Crowfoot P. D., Hunt A. L. The effect of oxygen tension on methylene hexadecanoic acid formation in Pseudomonas fluorescens and Escherichia coli. Biochim Biophys Acta. 1970 May 5;202(3):550–552. doi: 10.1016/0005-2760(70)90127-x. [DOI] [PubMed] [Google Scholar]
- ERWIN J., BLOCH K. BIOSYNTHESIS OF UNSATURATED FATTY ACIDS IN MICROORGANISMS. Science. 1964 Mar 6;143(3610):1006–1012. doi: 10.1126/science.143.3610.1006. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GORCHEIN A. ORNITHINE IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1964 Jun 15;84:356–358. doi: 10.1016/0926-6542(64)90064-2. [DOI] [PubMed] [Google Scholar]
- Gorchein A. Studies on the structure of an ornithine-containing lipid from non-sulphur purple bacteria. Biochim Biophys Acta. 1968 Mar 4;152(2):358–367. doi: 10.1016/0005-2760(68)90044-1. [DOI] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Deenen L. L. Changes in the chemical and the barrier properties of the membrane lipids of E. coli by variation of the temperature of growth. Chem Phys Lipids. 1969 Dec;3(4):413–417. doi: 10.1016/0009-3084(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Harris P., James A. T. The effect of low temperatures on fatty acid biosynthesis in plants. Biochem J. 1969 Apr;112(3):325–330. doi: 10.1042/bj1120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Bloch K. Beta-hydroxydecanoyl thioester dehydrase. Studies on molecular structure and active site. J Biol Chem. 1969 Nov 10;244(21):6014–6022. [PubMed] [Google Scholar]
- INGRAHAM J. L. Growth of psychrophilic bacteria. J Bacteriol. 1958 Jul;76(1):75–80. doi: 10.1128/jb.76.1.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M., HAGEN P. O. INFLUENCE OF TEMPERATURE ON FATTY ACID COMPOSITION OF PSYCHROPHILIC AND MESOPHILIC SERRATIA SPECIES. Can J Biochem. 1964 Apr;42:481–488. doi: 10.1139/o64-055. [DOI] [PubMed] [Google Scholar]
- Kass L. R., Brock D. J., Bloch K. Beta-hydroxydecanoyl thioester dehydrase. I. Purification and properties. J Biol Chem. 1967 Oct 10;242(19):4418–4431. [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Fatty acid synthesis in Escherichia coli. Biochem J. 1967 May;103(2):299–306. doi: 10.1042/bj1030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knivett V. A., Cullen J. Some factors affecting cyclopropane acid formation in Escherichia coli. Biochem J. 1965 Sep;96(3):771–776. doi: 10.1042/bj0960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J., Sturtevant J. M., Tsong T. Y. Characterization of the plasma membrane of Mycoplasma laidlawii. VII. Phase transitions of membrane lipids. Biochim Biophys Acta. 1970;219(1):114–122. doi: 10.1016/0005-2736(70)90066-0. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- SCHEURBRANDT G., BLOCH K. Unsaturated fatty acids in microorganisms. J Biol Chem. 1962 Jul;237:2064–2068. [PubMed] [Google Scholar]
- Shaw M. K., Ingraham J. L. Fatty Acid Composition of Escherichia coli as a Possible Controlling Factor of the Minimal Growth Temperature. J Bacteriol. 1965 Jul;90(1):141–146. doi: 10.1128/jb.90.1.141-146.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WALKER B. L., KUMMEROW F. A. ERYTHROCYTE FATTY ACID COMPOSITION AND APPARENT PERMEABILITY TO NON-ELECTROLYTES. Proc Soc Exp Biol Med. 1964 Apr;115:1099–1103. doi: 10.3181/00379727-115-29126. [DOI] [PubMed] [Google Scholar]
- ZALKIN H., LAW J. H., GOLDFINE H. Enzymatic synthesis of cyclopropane fatty acids catalyzed by bacterial extracts. J Biol Chem. 1963 Apr;238:1242–1248. [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]


