Abstract
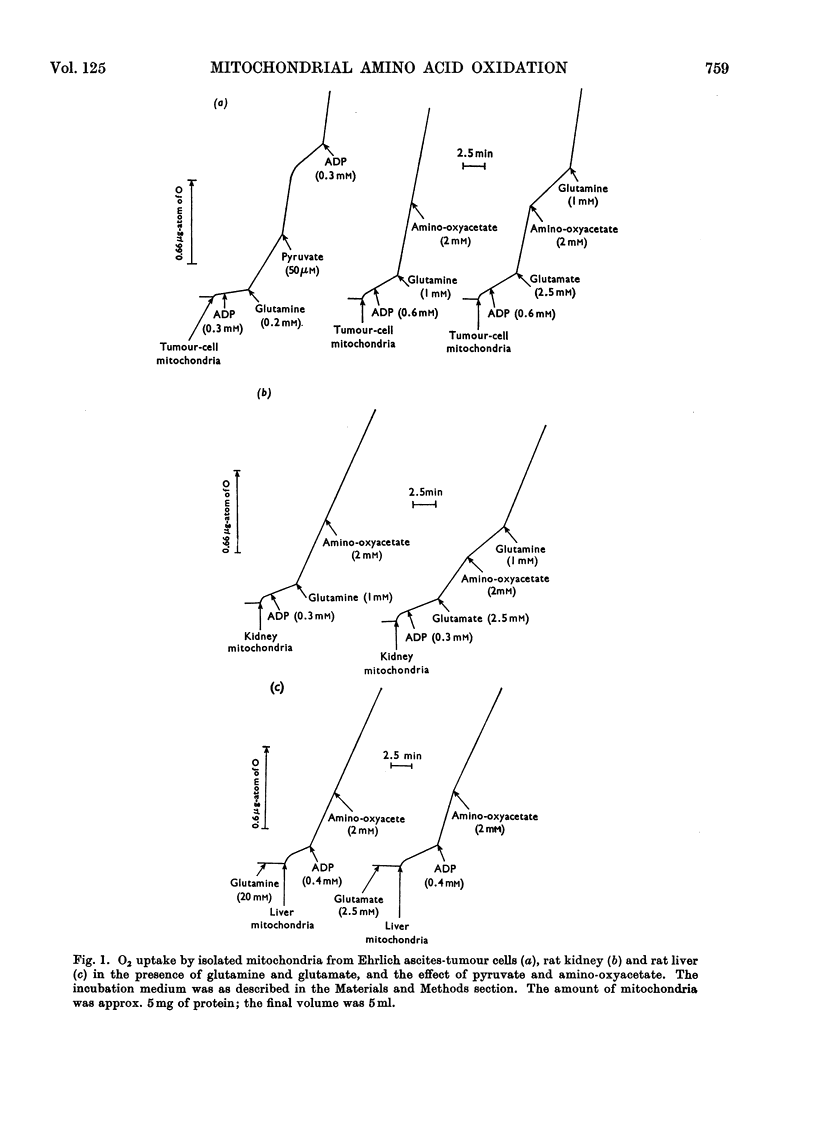
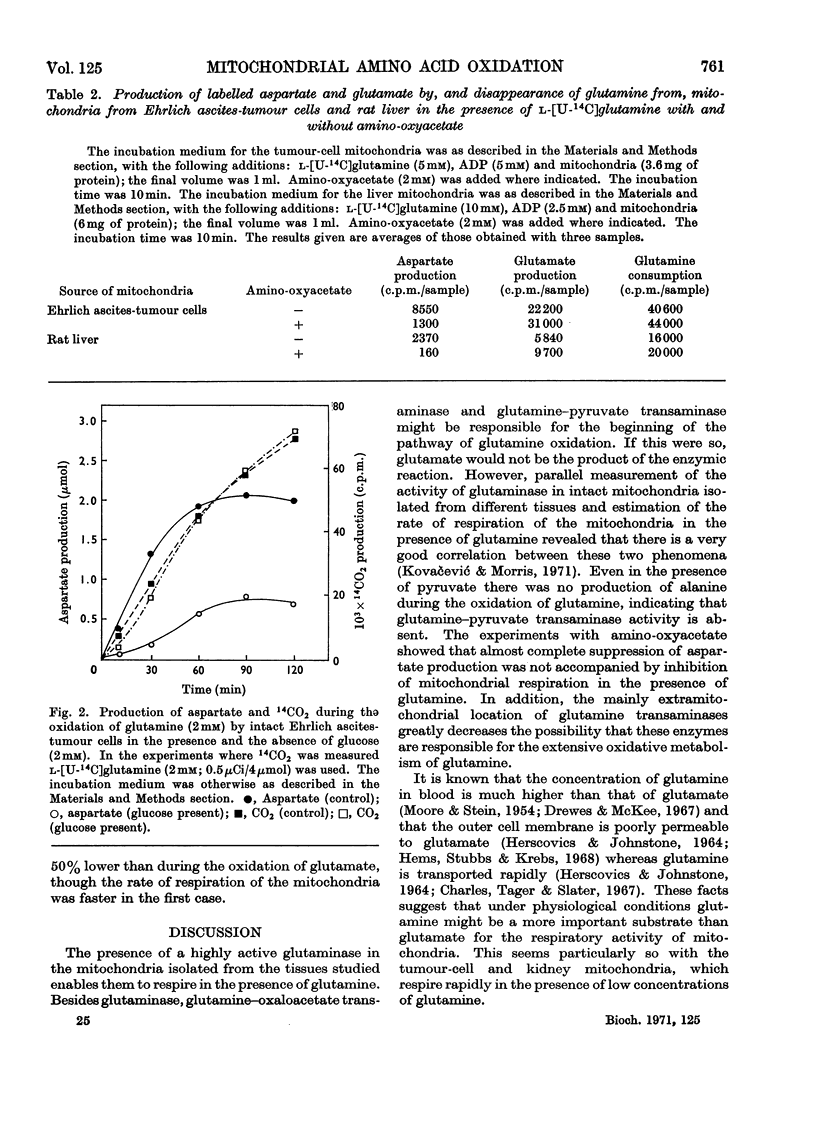
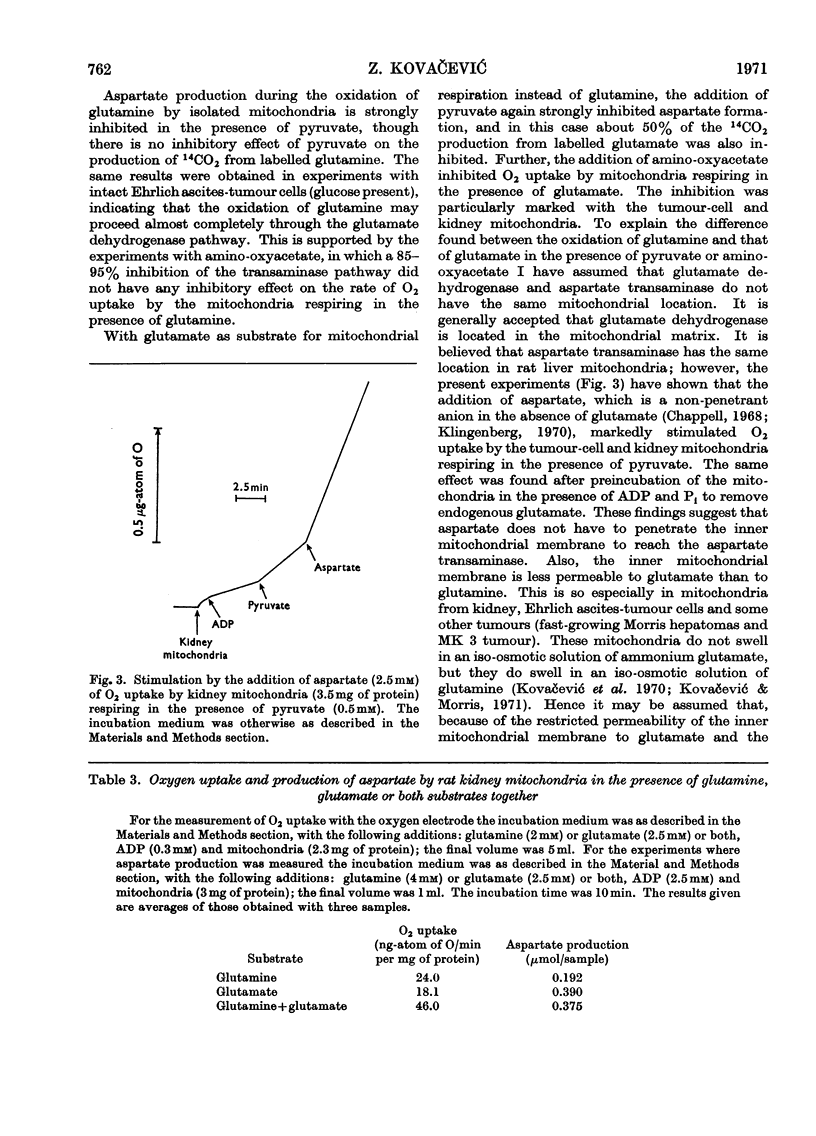
1. Pyruvate strongly inhibited aspartate production by mitochondria isolated from Ehrlich ascites-tumour cells, and rat kidney and liver respiring in the presence of glutamine or glutamate; the production of 14CO2 from l-[U-14C]glutamine was not inhibited though that from l-[U-14C]glutamate was inhibited by more than 50%. 2. Inhibition of aspartate production during glutamine oxidation by intact Ehrlich ascites-tumour cells in the presence of glucose was not accompanied by inhibition of CO2 production. 3. The addition of amino-oxyacetate, which almost completely suppressed aspartate production, did not inhibit the respiration of the mitochondria in the presence of glutamine, though the respiration in the presence of glutamate was inhibited. 4. Glutamate stimulated the respiration of kidney mitochondria in the presence of glutamine, but the production of aspartate was the same as that in the presence of glutamate alone. 5. The results suggest that the oxidation of glutamate produced by the activity of mitochondrial glutaminase can proceed almost completely through the glutamate dehydrogenase pathway if the transamination pathway is inhibited. This indicates that the oxidation of glutamate is not limited by a high [NADPH]/[NADP+] ratio. 6. It is suggested that under physiological conditions the transamination pathway is a less favourable route for the oxidation of glutamate (produced by hydrolysis of glutamine) in Ehrlich ascites-tumour cells, and perhaps also kidney, than the glutamate dehydrogenase pathway, as the production of acetyl-CoA strongly inhibits the first mechanism. The predominance of the transamination pathway in the oxidation of glutamate by isolated mitochondria can be explained by a restricted permeability of the inner mitochondrial membrane to glutamate and by a more favourable location of glutamate–oxaloacetate transaminase compared with that of glutamate dehydrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALAZS R. CONTROL OF GLUTAMATE OXIDATION IN BRAIN AND LIVER MITOCHONDRIAL SYSTEMS. Biochem J. 1965 May;95:497–508. doi: 10.1042/bj0950497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORST P., SLATER E. C. The oxidation of glutamate by rat-heart sarcosomes. Biochim Biophys Acta. 1960 Jun 17;41:170–171. doi: 10.1016/0006-3002(60)90391-7. [DOI] [PubMed] [Google Scholar]
- BORST P. The pathway of glutamate oxidation by mitochondria isolated from different tissues. Biochim Biophys Acta. 1962 Feb 26;57:256–269. doi: 10.1016/0006-3002(62)91119-8. [DOI] [PubMed] [Google Scholar]
- BRAUNSTEIN A. E. Les voices principales de l'assimilation et dissimilation de l'azote chez les animaux. Adv Enzymol Relat Subj Biochem. 1957;19:335–389. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes P. A., McKee R. W. Amino-acid composition of ascitic fluid and blood plasma from mice bearing Ehrlich-Lettre tumour. Nature. 1967 Jan 28;213(5074):411–412. doi: 10.1038/213411a0. [DOI] [PubMed] [Google Scholar]
- GUHA S. R. Intracellular localization of glutaminase I in rat liver. Enzymologia. 1961 May 15;23:94–100. [PubMed] [Google Scholar]
- HERSCOVICS A., JOHNSTONE R. M. MECHANISM OF ACTION OF GLUCOSE AND PYRIMIDINE NUCLEOSIDES ON (14C)FORMATE UTILIZATION BY EHRLICH ASCITES CELLS. Biochim Biophys Acta. 1964 Nov 15;91:365–373. doi: 10.1016/0926-6550(64)90065-9. [DOI] [PubMed] [Google Scholar]
- Hems R., Stubbs M., Krebs H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968 May;107(6):807–815. doi: 10.1042/bj1070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird F. J., Marginson M. A. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968 Sep 20;127(1):718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BELLAMY D. The interconversion of glutamic acid and aspartic acid in respiring tissues. Biochem J. 1960 Jun;75:523–529. doi: 10.1042/bj0750523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Mitochondria metabolite transport. FEBS Lett. 1970 Feb 16;6(3):145–154. doi: 10.1016/0014-5793(70)80044-8. [DOI] [PubMed] [Google Scholar]
- Kovacević Z., McGivan J. D., Chappell J. B. Conditions for activity of glutaminase in kidney mitochondria. Biochem J. 1970 Jun;118(2):265–274. doi: 10.1042/bj1180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN W. H., MOORE S. The free amino acids of human blood plasma. J Biol Chem. 1954 Dec;211(2):915–926. [PubMed] [Google Scholar]
- Vinogradov A. D. Vliianie alpha-ketogliutarata i ionov ammoniia na okislenie suktsinata mitokhondriiami pecheni. Biokhimiia. 1968 May-Jun;33(3):553–560. [PubMed] [Google Scholar]
- WALLACH D. P. Studies on the GABA pathway. I. The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid). Biochem Pharmacol. 1961 Feb;5:323–331. doi: 10.1016/0006-2952(61)90023-5. [DOI] [PubMed] [Google Scholar]


