Abstract
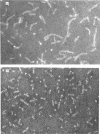
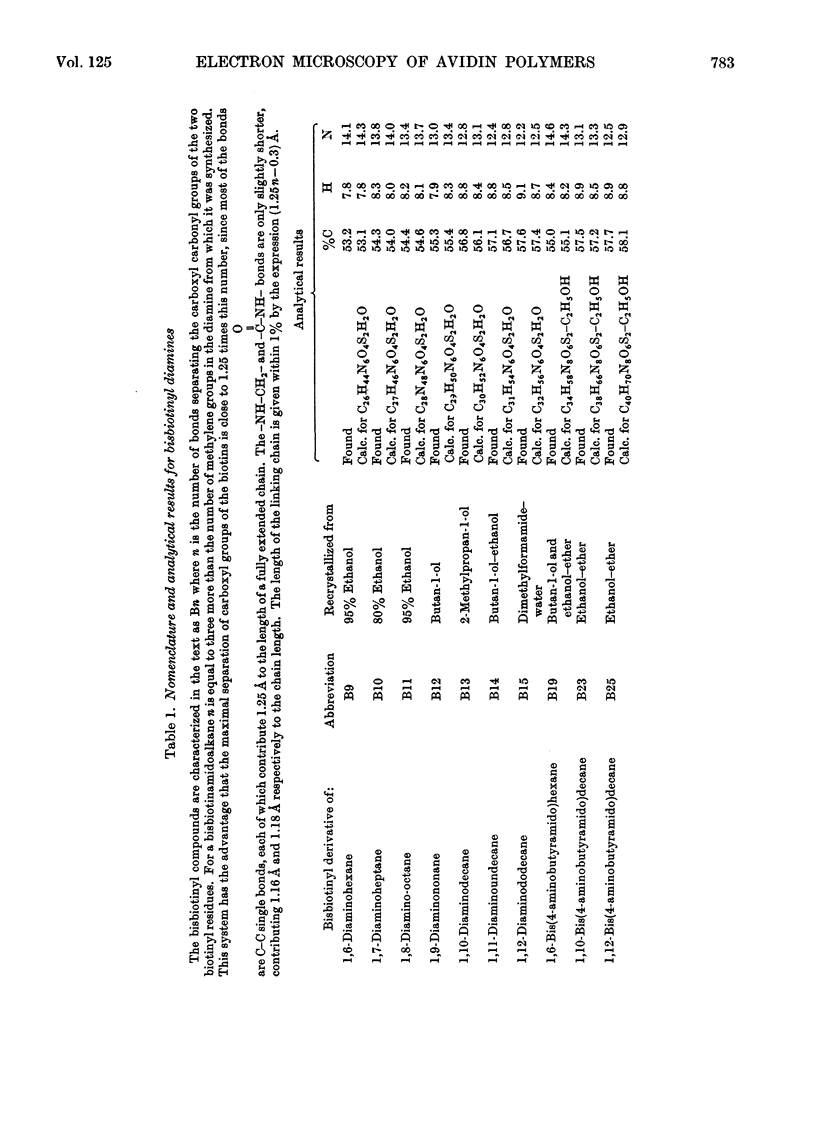
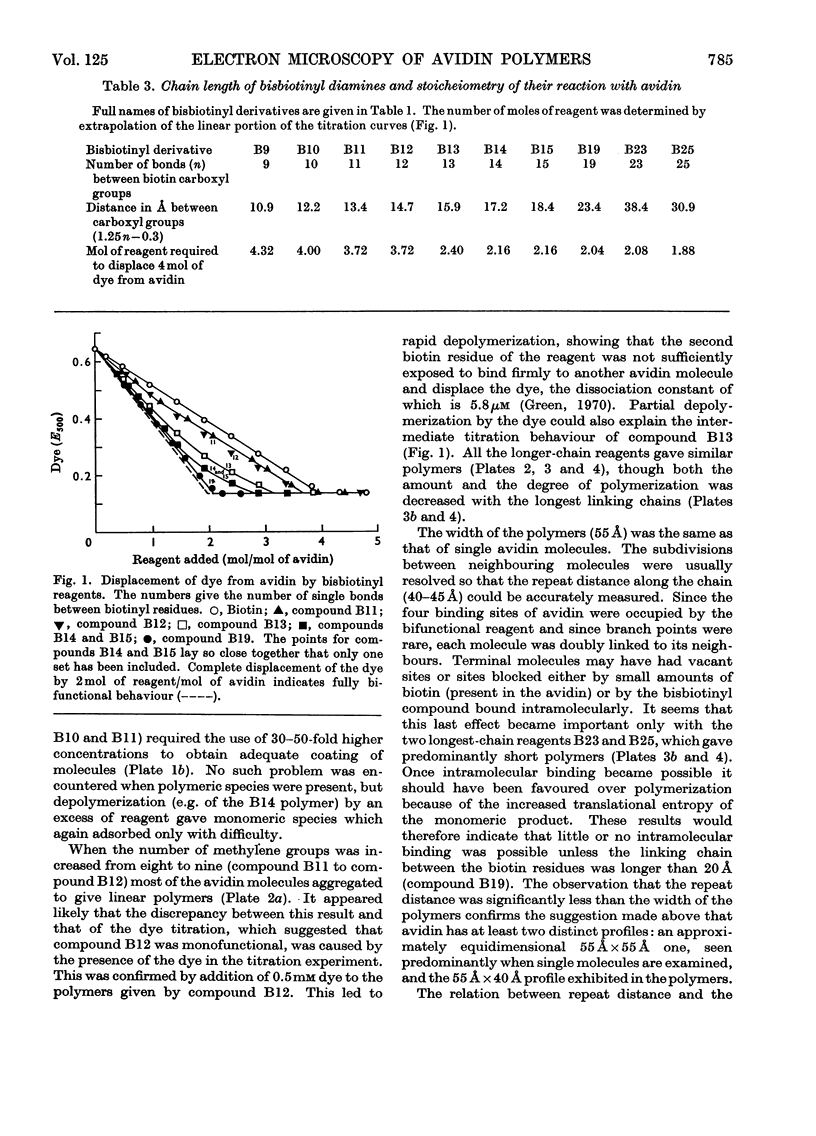
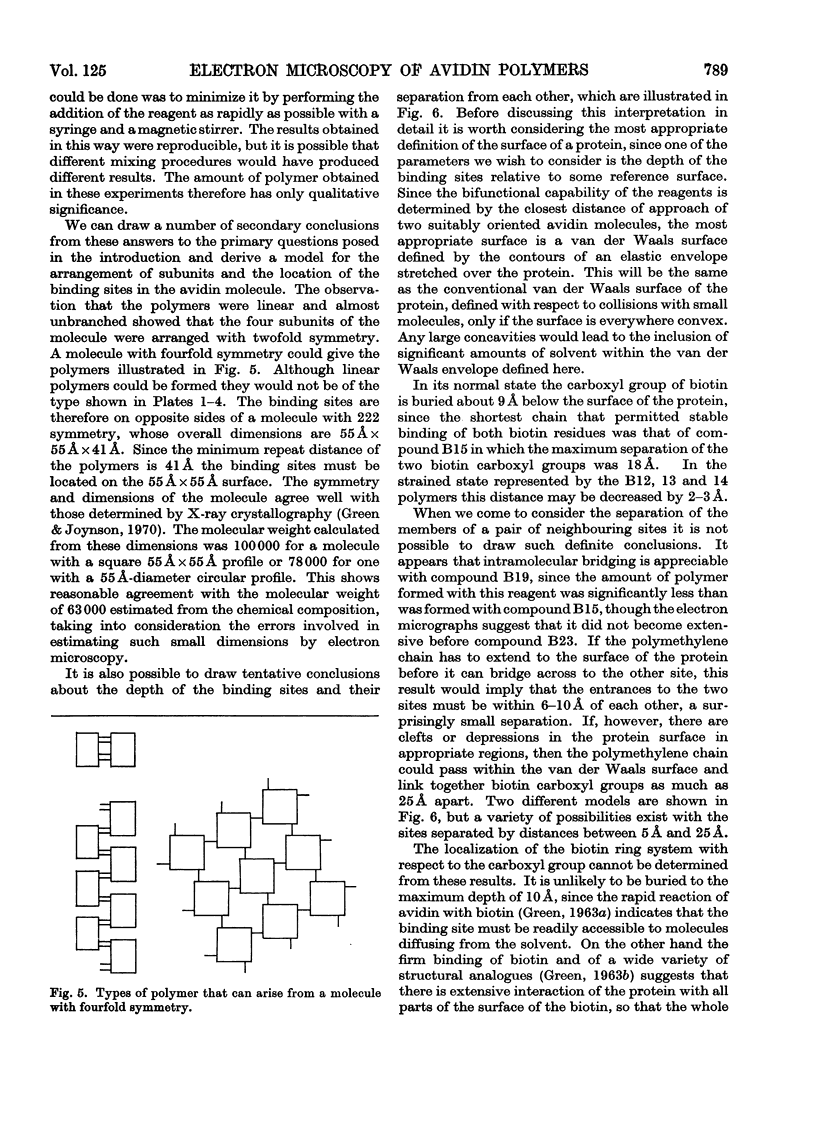
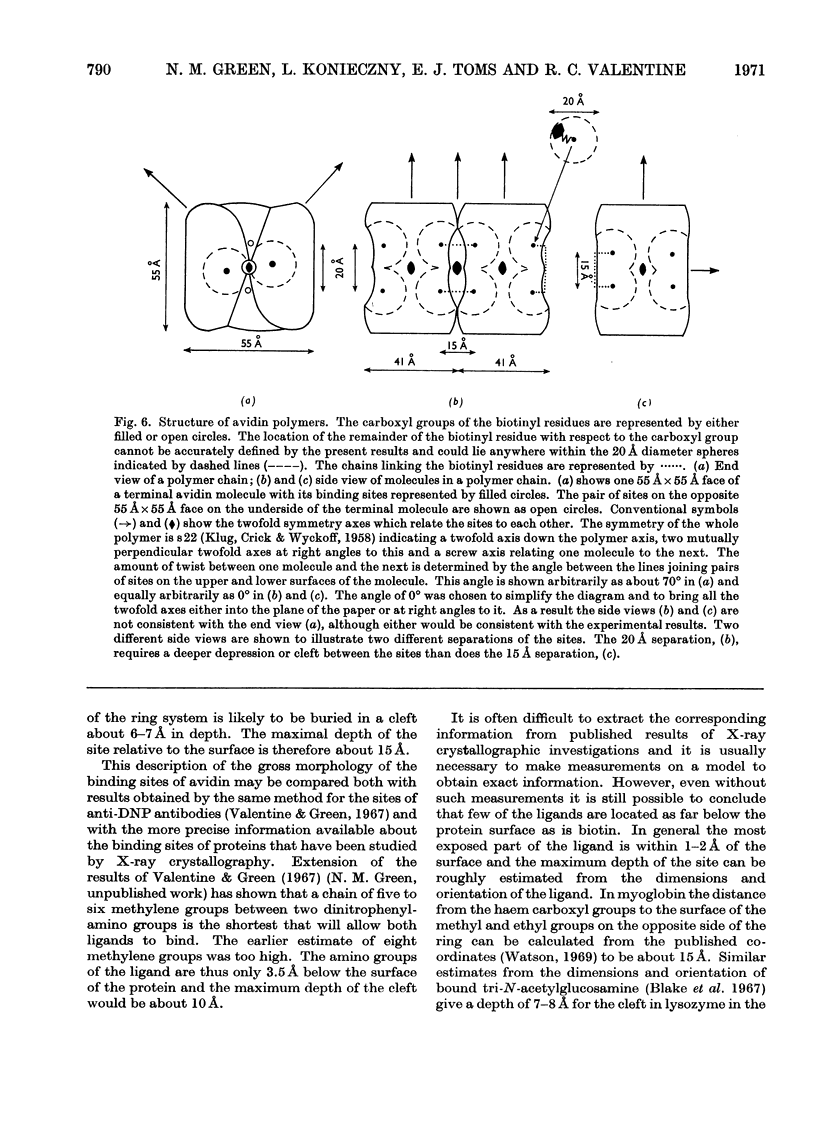
A series of bisbiotinyl diamines was synthesized with between 9 and 25 bonds between the carboxyl groups of the two biotin residues. It was found that only one of the two biotin residues could combine with avidin when there were fewer than 12 bonds between the biotin residues. Compounds with longer chains behaved in a bifunctional manner and gave rise to linear polymers of avidin, which were characterized by electron microscopy and by gel filtration. The polymers formed with the shorter-chain reagents (12, 13 or 14 bonds) were relatively unstable and could be depolymerized by weakly bound analogues of biotin. The polymers of longer-chain reagents were not depolymerized under these conditions and were only slowly affected by added biotin. When the chain length of the reagent reached 23 bonds the polymers became much shorter, suggesting that the reagent was now able to link two subunits within the same avidin molecule. From the morphology of the polymers it could be concluded that the four subunits of the avidin molecules were arranged with 222 symmetry and that they were grouped in two pairs at opposite ends of the short axis of the molecule whose dimensions were 55Å×55Å×41Å.
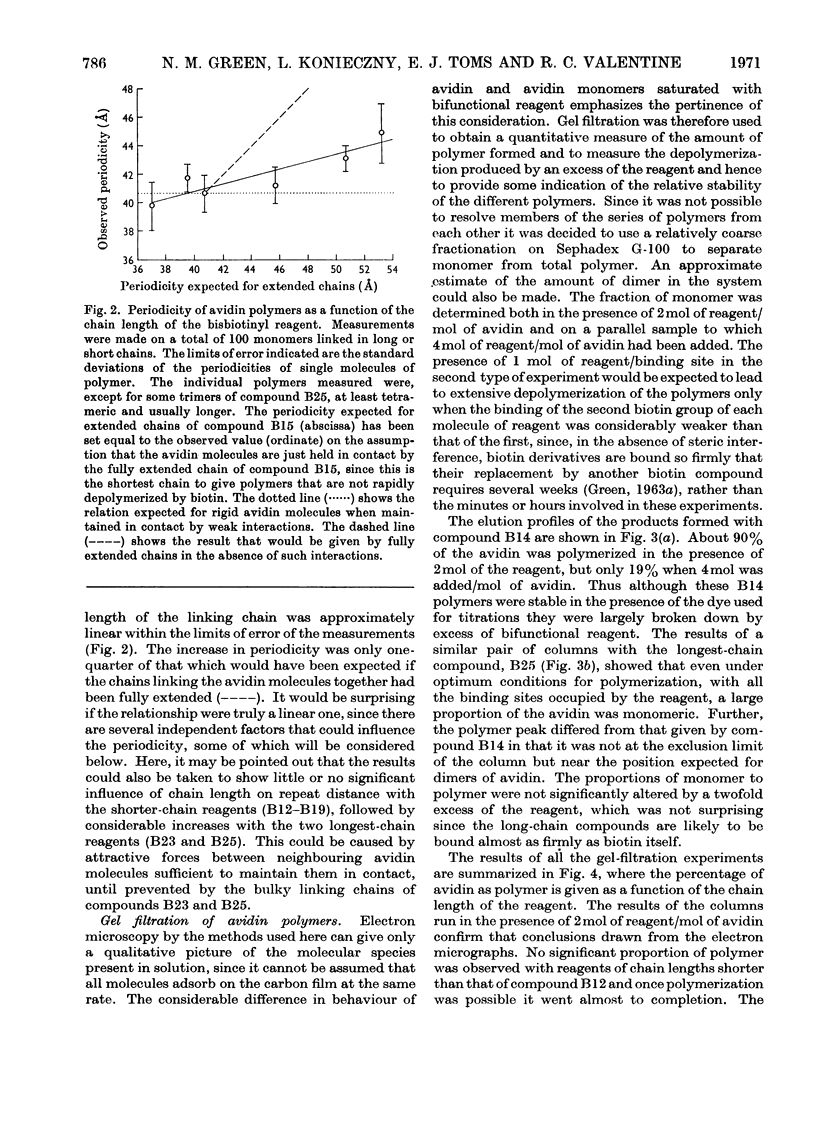
Full text
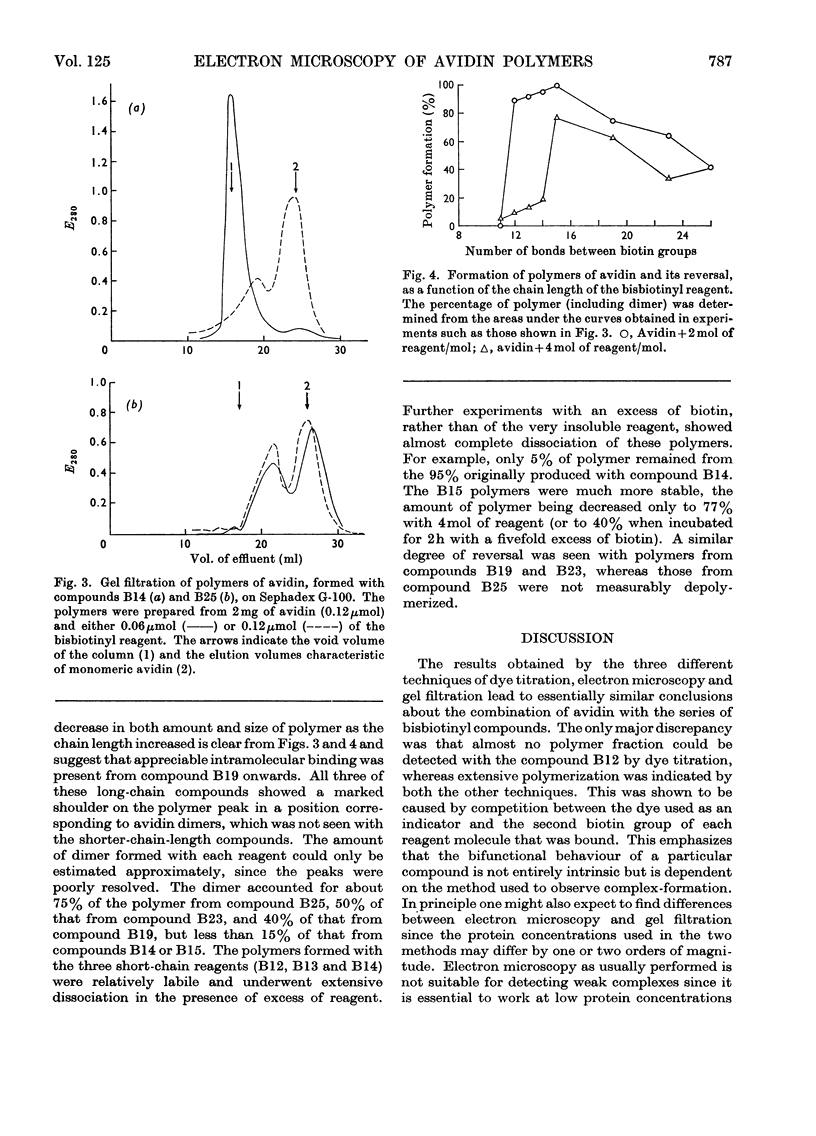
PDF


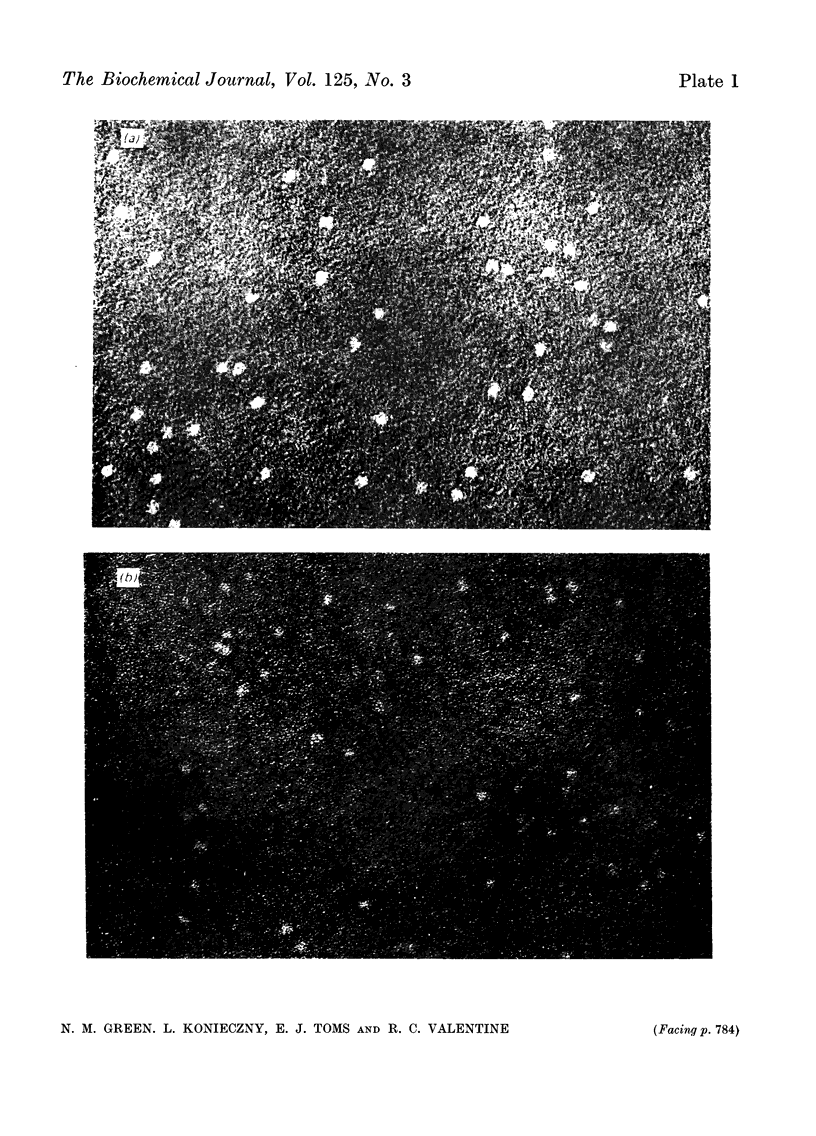
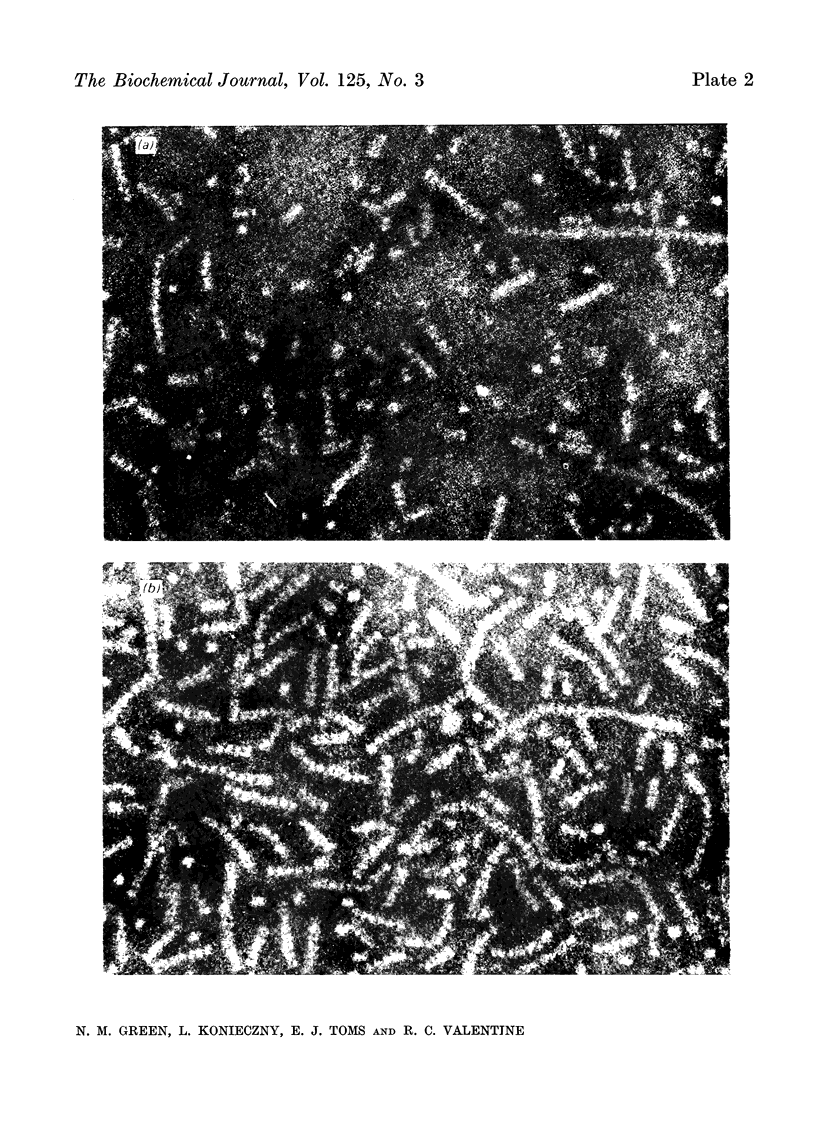
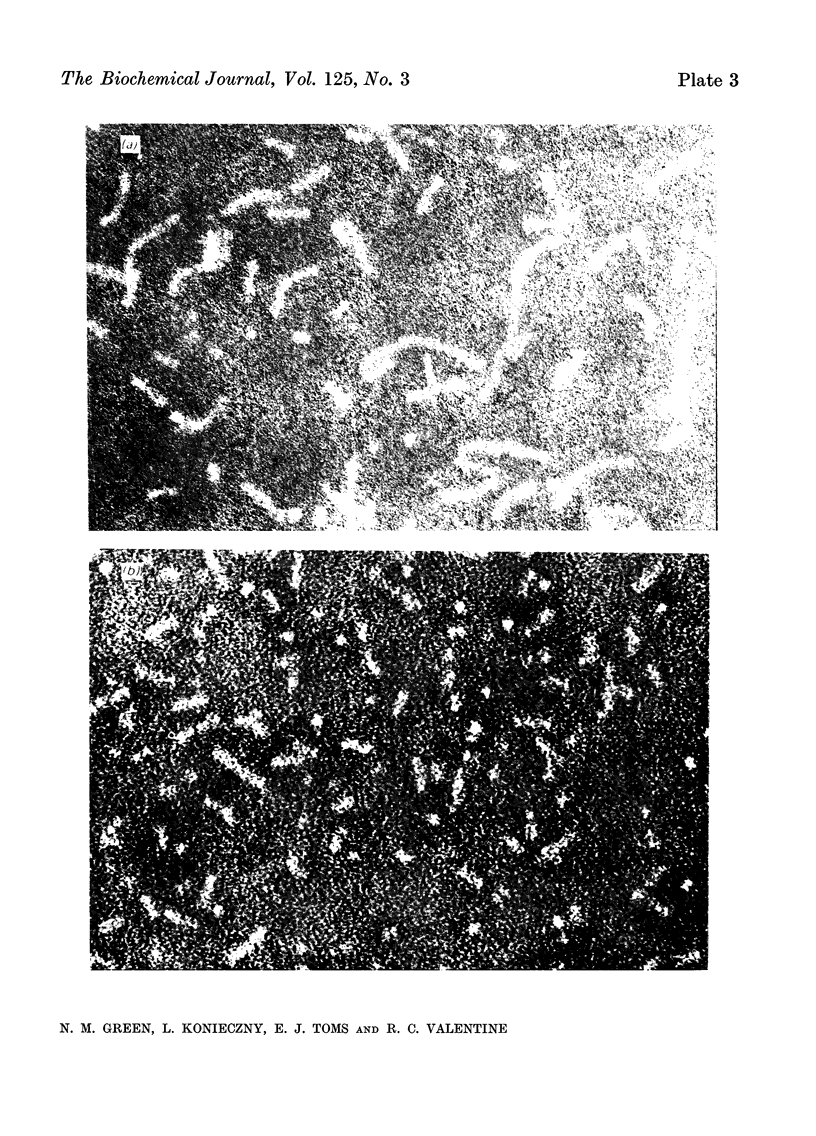



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- DeLange R. J. Egg white avidin. I. Amino acid composition; sequence of the amino- and carboxyl-terminal cyanogen bromide peptides. J Biol Chem. 1970 Mar 10;245(5):907–916. [PubMed] [Google Scholar]
- DeLange R. J., Huang T. S. Egg white avidin. 3. Sequence of the 78-residue middle cyanogen bromide peptide. Complete amino acid sequence of the protein subunit. J Biol Chem. 1971 Feb 10;246(3):698–709. [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 1. THE USE OF (14-C)BIOTIN FOR KINETIC STUDIES AND FOR ASSAY. Biochem J. 1963 Dec;89:585–591. doi: 10.1042/bj0890585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 3. THE NATURE OF THE BIOTIN-BINDING SITE. Biochem J. 1963 Dec;89:599–609. doi: 10.1042/bj0890599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M., Joynson M. A. A preliminary crystallographic investigation of avidin. Biochem J. 1970 Jun;118(1):71–72. doi: 10.1042/bj1180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M., Toms E. J. Purification and crystallization of avidin. Biochem J. 1970 Jun;118(1):67–70. doi: 10.1042/bj1180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon G. B. On the interpretation of high resolution electron micrographs of macromolecules. J Ultrastruct Res. 1968 Dec;25(5):349–361. doi: 10.1016/s0022-5320(68)80091-7. [DOI] [PubMed] [Google Scholar]
- KNAPPE J., BRUEMMER W., BIEDERBICK K. REINIGUNG UND EIGENSCHAFTEN DER BIOTINIDASE AUS SCHWEINENIEREN UND LACTOBACILLUS CASEI. Biochem Z. 1963;338:599–613. [PubMed] [Google Scholar]
- MELAMED M. D., GREEN N. M. AVIDIN. 2. PURIFICATION AND COMPOSITION. Biochem J. 1963 Dec;89:591–599. doi: 10.1042/bj0890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELAMED M. D., GREEN N. M. AVIDIN. 2. PURIFICATION AND COMPOSITION. Biochem J. 1963 Dec;89:591–599. doi: 10.1042/bj0890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- Ryder E., Gregolin C., Chang H. C., Lane M. D. Liver acetyl CoA carboxylase: insight into the mechanism of activation by tricarboxylic acids and acetyl CoA. Proc Natl Acad Sci U S A. 1967 May;57(5):1455–1462. doi: 10.1073/pnas.57.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]