Abstract
Background
Anthracyclines, a highly effective chemotherapy for many pediatric malignancies, cause cardiomyopathy, a major late effect in adult survivors. Biomarkers are needed for early detection and targeted interventions for anthracycline-associated cardiomyopathy.
Objectives
The aim of this study was to determine if serum proteins and/or metabolites in asymptomatic childhood cancer survivors can discriminate symptomatic cardiomyopathy.
Methods
Using an untargeted mass spectrometry–based approach, 867 proteins and 218 metabolites were profiled in serum samples of 75 asymptomatic survivors with subclinical cardiomyopathy and 75 individually matched survivors without cardiomyopathy from SJLIFE (St. Jude Lifetime Cohort Study). Models were developed on the basis of the most influential differentially expressed proteins and metabolites, using conditional logistic regression with a least absolute shrinkage and selection operator penalty. The best performing model was evaluated in 23 independent survivors with severe or symptomatic cardiomyopathy and 23 individually matched cardiomyopathy-free survivors.
Results
A 27-protein model identified using conditional logistic regression with a least absolute shrinkage and selection operator penalty discriminated symptomatic or severe cardiomyopathy requiring heart failure medications in independent survivors; 19 of 23 individually matched survivors with and without cardiomyopathy were correctly discriminated with 82.6% (95% CI: 71.4%-93.8%) accuracy. Pathway enrichment analysis revealed that the 27 proteins were enriched in various biological processes, many of which have been linked to anthracycline-related cardiomyopathy.
Conclusions
A risk model was developed on the basis of the differential expression of serum proteins in subclinical cardiomyopathy, which accurately discriminated the risk for severe cardiomyopathy in an independent, matched sample. Further assessment of these proteins as biomarkers of cardiomyopathy risk should be conducted in external larger cohorts and through prospective studies.
Key Words: anthracycline, biomarkers, cancer survivorship, childhood cancer, metabolomics, proteomics
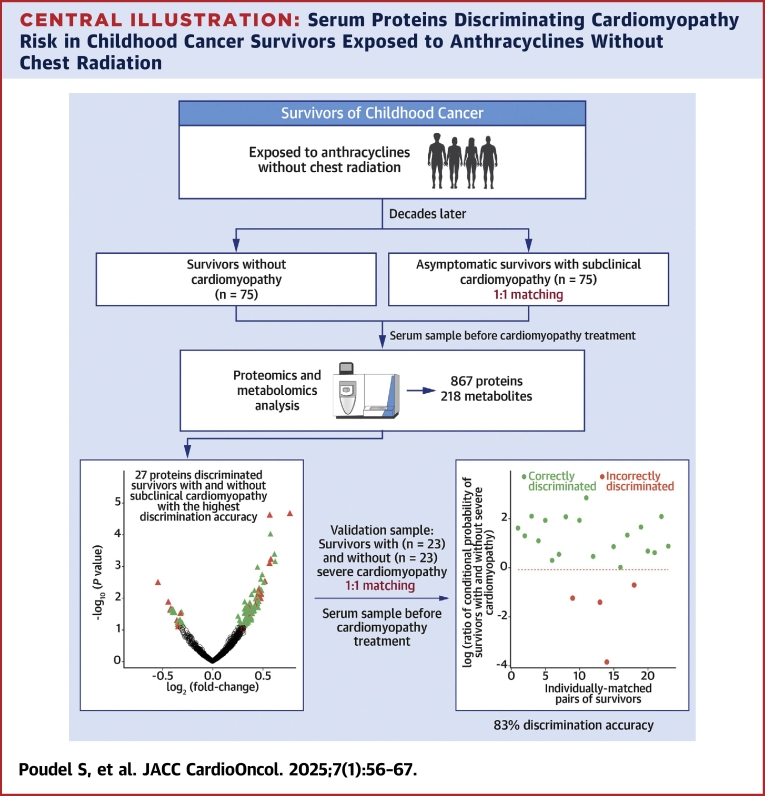
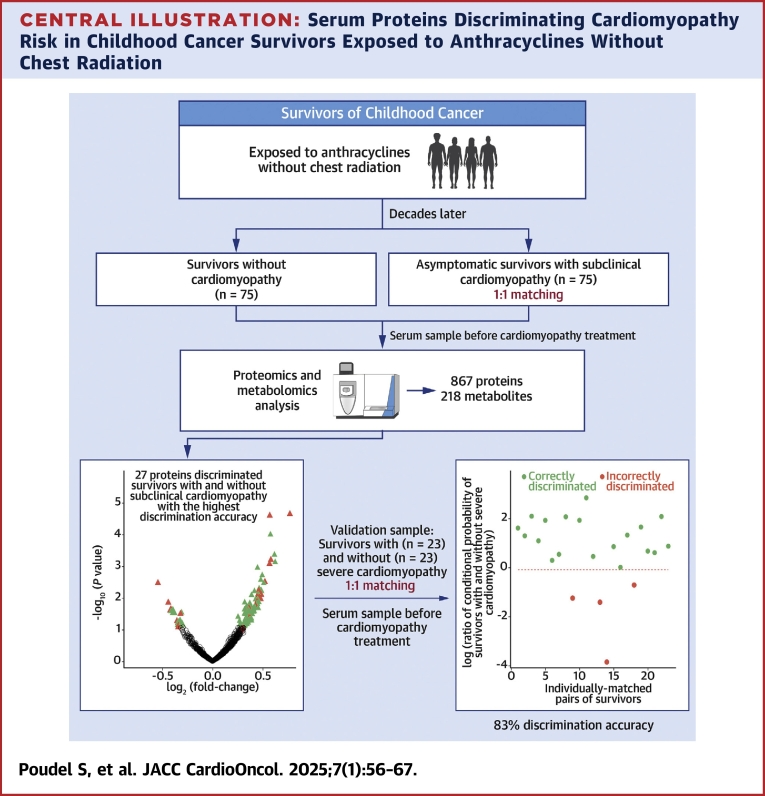
Central Illustration
Anthracyclines are a highly effective class of chemotherapeutic agents used in approximately 60% of pediatric patients with solid and hematological malignancies.1,2 However, the use of anthracyclines is complicated by a well-established, dose-dependent risk for heart failure. Compared with survivors not exposed to anthracycline chemotherapy, those with cumulative exposures of 100 to 250 mg/m2 are at 2-fold, and those exposed to ≥250 mg/m2 at nearly 5-fold, increased risk for heart failure.3,4 Once a patient is diagnosed with heart failure, the prognosis is poor, with some estimates of 5-year survival rate <50%.5,6 Before presenting with clinically overt signs and symptoms of heart failure, survivors exposed to anthracyclines often develop subclinical changes in left ventricular systolic function with a decrease in ejection fraction (EF). Recognizing the high risk for cardiac dysfunction associated with anthracycline exposure, surveillance guidelines have recommended routine echocardiography assessing EF for cardiotoxicity-exposed survivors for early detection and potential intervention.7 However, EF has poor sensitivity for detecting subtle changes in cardiac function and may demonstrate intrapatient and interobserver variability.8 A decline in EF may be evident on imaging modalities only after significant cardiac dysfunction has developed, which can be irreversible and becomes refractory to pharmacologic intervention.9 Improved methods of early detection are needed.
Circulating biomarkers may serve as screening tools for the early detection of cardiomyopathy, potentially reducing the need for extensive echocardiographic evaluations. They may also be used in combination with imaging modalities to further improve diagnostic accuracy. Most studies that have evaluated serum biomarkers, such as cardiac troponins and N-terminal pro–brain natriuretic peptide, to detect asymptomatic or early-stage cardiac dysfunction in long-term childhood cancer survivors10, 11, 12, 13, 14 have reported low sensitivity and high specificity. However, a recent study among survivors at moderate or high risk for cardiomyopathy14 identified a 2-fold increased risk on the basis of abnormal levels of N-terminal pro–brain natriuretic peptide and a 4-fold increased risk when combined with abnormal global longitudinal strain detected by echocardiography. Additional biomarkers for anthracycline-related cardiomyopathy have also been assessed; however, these results are not yet validated in independent samples.15,16
In this study, we used an untargeted approach to profile serum proteins and metabolites in long-term survivors of childhood cancer exposed to anthracyclines without chest radiation from SJLIFE (St. Jude Lifetime Cohort Study). A risk discrimination model based on the most influential biomarkers was developed to discriminate the risk for subclinical cardiomyopathy among asymptomatic survivors and its ability to discriminate the risk for severe cardiomyopathy (requiring heart failure medications) was evaluated in an independent set of symptomatic survivors.
Methods
Study population and design
Participants were sampled from SJLIFE. The cohort design and participant recruitment of SJLIFE have previously been described.17,18 Briefly, SJLIFE, initiated in 2007, is a retrospectively constructed cohort study with prospective clinical follow-up and ongoing enrollment of survivors of childhood cancer treated from 1962 to 2012 and followed at St. Jude Children’s Research Hospital. A comprehensive clinical assessment of health conditions among SJLIFE participants was performed, which included echocardiography with severity grading on the basis of a modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.19 For this study, 98 survivors with cardiomyopathy were individually matched in a 1:1 ratio with 98 cardiomyopathy-free survivors on the basis of sex, primary cancer type, race/ethnicity, and age at cancer diagnosis (≥10 years vs <10 years). Considering the well-established association of anthracycline exposure with risk for cardiomyopathy that increases with age, cardiomyopathy-free (resting EF ≥50%) survivors were also required to have received the same minimum level of anthracycline exposure and be older at the time of cardiomyopathy assessment compared with their matched survivors with cardiomyopathy. When multiple cardiomyopathy-free survivors met these criteria, the survivor with the closest anthracycline exposure and age at cardiomyopathy assessment to the matched survivor with cardiomyopathy was selected. All 196 survivors were exposed to anthracyclines without chest radiation exposure. Of the 98 survivors with cardiomyopathy, 75 were asymptomatic and had subclinical cardiomyopathy (CTCAE grade 2; resting EF 40%-49% or 10%-19% absolute decrease from baseline), while 23 were symptomatic and developed severe cardiomyopathy (CTCAE grade 3; resting EF 20%-39% or >20% absolute decline from baseline or requiring heart failure medications).
Biomarkers for asymptomatic cardiomyopathy that inform the risk for symptomatic cardiomyopathy may provide insight into early mechanisms as well as opportunities for early detection, prior to severe cardiomyopathy and targeted interventions. Thus, we considered asymptomatic survivors with subclinical cardiomyopathy and their matched cardiomyopathy-free survivors as our discovery sample. Symptomatic survivors with severe cardiomyopathy and their matched cardiomyopathy-free survivors were included in the validation sample. A serum sample was obtained at a SJLIFE campus visit. For survivors with cardiomyopathy, serum was analyzed in the sample from either the SJLIFE visit prior to cardiomyopathy diagnosis (47% in discovery and 65% in validation samples) or the sample from the visit at which cardiomyopathy was discovered (ie, pretreatment for cardiomyopathy). The study was approved by the Institutional Review Board of St. Jude Children’s Research Hospital, and all participants provided written informed consent.
Cancer therapy exposures
Information pertaining to exposures to chemotherapy and radiotherapy was abstracted systematically from medical records. The cumulative anthracycline dose (milligrams per square meter) was determined by doxorubicin toxicity equivalence.20
Proteome and metabolome profiling
Untargeted proteomics and metabolomics experiments were performed on the same serum sample of each survivor. The samples were distributed across 14 distinct batches and randomized within each batch, using a single-blinded approach to minimize bias. Serum samples of each matched pair of survivors were included in the same batch. Proteome profiling was performed using an untargeted global approach with 16-plex isobaric tandem mass tag labeling reactions, 2-dimensional reversed-phase liquid chromatography fractionation, and tandem mass spectrometry. For each of the 196 survivor samples used for proteome profiling, metabolome profiling was carried out using liquid chromatography–tandem mass spectrometry. Detailed methods on the protein and metabolite measurements and raw data processing are described in the Supplemental Appendix.
Statistical analyses
Although the initial identification of proteins and metabolites used several libraries, for a more reliable assessment, we focused specifically on 867 known proteins (Supplemental Table 1) and 218 metabolites (Supplemental Table 2) identified by our in-house library. Prior to the analyses, protein and metabolite values were log2-transformed, and each protein or metabolite was normalized (mean = 0, SD = 1). In the discovery sample, differential expression of proteins and metabolites between asymptomatic survivors with and without subclinical cardiomyopathy was assessed using a linear mixed-effects model (lmer function in the R package lme421), adjusted for age at primary cancer diagnosis, sex, race/ethnicity, age at serum sampling, and cumulative dose of anthracyclines as fixed effects and matched-pair indicator as a random effect. Primary cancer type was not used for data adjustment, because of its correlation with cancer treatment, including cumulative anthracycline dose. The resulting P values were corrected for multiple testing using the Benjamini-Hochberg procedure,22 and a false discovery rate (FDR) of <0.25 was considered as suggestive of statistical significance.
The assumptions of the linear mixed-effects model were examined for the proteins selected in the final model using the following methods. First, we checked the linearity assumption for each protein by analyzing the relationship between the normalized serum protein level and each continuous covariate (age at primary cancer diagnosis, cumulative anthracycline doses, and age at serum sampling). Second, we assessed the normality assumption by examining the relationship between the fitted values and residuals for each protein. Last, we further evaluated the normality assumption of the residuals using quantile-quantile plots.
Risk discrimination models for subclinical cardiomyopathy were developed with the discovery sample on the basis of the most influential proteins or metabolites using conditional logistic regression with a least absolute shrinkage and selection operator penalty (CLR-Lasso) conditioned on the matched-pair indicator. Top (5%, 10%, 15%, 20%, and 25%) proteins and metabolites on the basis of the lowest P values from the differential expression analyses of the discovery sample were entered into the CLR-Lasso model as candidate predictors. Separate models were built using proteins and metabolites. The shrinkage (lambda) parameter in CLR-Lasso was selected using 10-fold cross-validation in the R package clogitL1.23 Proteins or metabolites with nonzero beta coefficients were selected as predictors. Under the study design, only 1 survivor per matched pair developed cardiomyopathy, and therefore a survivor having higher predicted conditional probability within the pair was labeled as “predicted to be with cardiomyopathy” and the other matched survivor was labeled as “predicted to be cardiomyopathy free.” The discrimination accuracy was assessed by the concordance between predicted and observed cardiomyopathy status per matched pair. The model with the highest discriminatory accuracy in the discovery sample and the largest number of predictors selected by CLR-Lasso was declared as the best performing model of the discovery stage. The best performing model at the discovery stage was evaluated for its ability to discriminate the risk for severe cardiomyopathy in the validation sample. The corresponding 95% CI of the discrimination accuracy was calculated using 2,000 bootstrapped samples.
A standard calibration plot compares the predicted probabilities of a prediction model with the actual probabilities of the outcome. As we use a matched case-control design and our prediction model is based on a conditional logistic regression model, which does not provide event probabilities, the standard calibration plot, which assesses the predictive performance of a prediction model in a study sample obtained from random sampling, cannot be obtained. Thus, to illustrate the predictive performance of our best performing model, we used the following approach: for each matched pair in the validation sample, we calculated the predicted OR of cardiomyopathy on the basis of our model for the case vs the control within a matched pair. We compared these ORs between the concordant pairs (predicted OR: >1) and discordant pairs (predicted OR: <1): a good calibration may show large ORs for the concordant pairs (ie, the degree of correctness in prediction is appreciable) and <1.0 but closer to 1.0 for the discordant pairs (ie, the degree of incorrectness in prediction is not appreciable).
Post hoc analyses of candidate biomarkers
We conducted Gene Ontology (GO) enrichment analysis on a set of proteins included in the best performing model and those that were consistently differentially expressed in both discovery and validation samples using g:Profiler.24 Homo sapiens was specified as the organism, and we applied the g:Profiler-specific g:SCS algorithm for multiple testing correction (for GO terms) with a statistical significance threshold of 0.05, while keeping all other parameters at default settings. Additionally, we performed protein-protein interaction (PPI) analysis on the same candidate biomarkers using the STRING database (https://string-db.org).25 We considered all available interaction sources and set a minimum required interaction score of medium confidence (0.40). To visualize the comprehensive PPI network beyond the selected molecules, we included all 867 proteins identified by our in-house libraries and used stringApp version 2.0.126 within the Cytoscape platform,25 importing functional associations and PPI information from the String PPI database.27
Results
Clinical characteristics of the survivors in the discovery and validation samples were comparable (Table 1): the majority were men (61.3% and 69.6%, respectively) and self-reported as White (89.3% and 82.6%, respectively). In the discovery sample, 98.7% of survivors with cardiomyopathy and 94.7% without it were non-Hispanic. In the validation sample, these percentages were 100.0% and 95.7%, respectively. Nearly one-half were diagnosed with childhood cancer before 10 years of age. The median age at the detection of subclinical cardiomyopathy in the discovery sample was 32.2 years (range: 16.0-47.7 years) and of severe cardiomyopathy in the validation sample was 32.6 years (range: 11.2-46.7 years). The median time from serum sampling to cardiomyopathy was 0.01 years (range: 0.00-6.48 years) in the discovery sample and 0.92 years (range: 0.00-5.51 years) in the validation sample. On the basis of the applied matching criteria, the median cumulative anthracycline dose among survivors with cardiomyopathy was lower than among those without cardiomyopathy in both discovery (178.0 mg/m2 vs 243.0 mg/m2) and replication (175.1 mg/m2 vs 216.0 mg/m2) samples. The majority of survivors with and without cardiomyopathy were treated with doxorubicin and the others with daunorubicin, except for 2 survivors in the discovery sample who were exposed to mitoxantrone.
Table 1.
Clinical Characteristics of Study Participants From the St. Jude Lifetime Cohort Study
| Discovery Sample |
Validation Sample |
|||
|---|---|---|---|---|
| Subclinical Cardiomyopathy (n = 75) | Without Cardiomyopathy (n = 75) | Severe Cardiomyopathy (n = 23) | Without Cardiomyopathy (n = 23) | |
| Age at primary cancer diagnosis, y | ||||
| ≥10 | 33 (44.0) | 33 (44.0) | 12 (52.2) | 12 (52.2) |
| <10 | 42 (56.0) | 42 (56.0) | 11 (47.8) | 11 (47.8) |
| Sex | ||||
| Male | 46 (61.3) | 46 (61.3) | 16 (69.6) | 16 (69.6) |
| Female | 29 (38.7) | 29 (38.7) | 7 (30.4) | 7 (30.4) |
| Primary cancer diagnosis | ||||
| Acute lymphoblastic leukemia | 28 (37.3) | 28 (37.3) | 5 (21.7) | 5 (21.7) |
| Hodgkin lymphoma | 9 (12.0) | 9 (12.0) | 3 (13.0) | 3 (13.0) |
| Non-Hodgkin lymphoma | 9 (12.0) | 9 (12.0) | 3 (13.0) | 3 (13.0) |
| Osteosarcoma | 9 (12.0) | 9 (12.0) | 2 (8.7) | 2 (8.7) |
| Wilms’ tumor | 5 (6.7) | 5 (6.7) | 3 (13.0) | 3 (13.0) |
| Other cancers | 15 (20.0) | 15 (20.0) | 7 (30.4) | 7 (30.4) |
| Race | ||||
| White | 67 (89.3) | 67 (89.3) | 19 (82.6) | 19 (82.6) |
| Black | 8 (10.7) | 8 (10.7) | 4 (17.4) | 4 (17.4) |
| Ethnicity | ||||
| Non-Hispanic | 74 (98.7) | 71 (94.7) | 23 (100.0) | 22 (95.7) |
| Hispanic | 1 (1.3) | 4 (5.3) | 0 (0.0) | 1 (4.3) |
| Age at cardiomyopathy assessment, y | 32.2 (16.0-47.7) | 35.4 (16.1-52.7) | 32.6 (11.2-46.7) | 35.3 (18.0-51.2) |
| Cumulative anthracycline dose, mg/m2 | 178.0 (24.6-564.5) | 243.0 (44.8-694.7) | 175.1 (22.0-392.0) | 216.0 (51.3-497.4) |
| Exposure | ||||
| Doxorubicin | 52 (69.3) | 62 (82.7) | 19 (82.6) | 20 (87) |
| Daunorubicin | 28 (37.3) | 21 (28.0) | 6 (26.1) | 3 (13) |
| Mitoxantrone | 1 (1.3) | 1 (1.3) | 0 (0) | 0 (0) |
| Age at serum sample, y | 31.1 (15.9-43.8) | 36.5 (16.6-52.7) | 32.3 (10.2-45.9) | 36.5 (18.9-51.2) |
Values are n (%) or median (range).
In the discovery sample, 13 proteins were significantly differentially expressed (FDR <0.25) among asymptomatic survivors with subclinical cardiomyopathy (Supplemental Figure 1, Supplemental Table 3). Of these, 12 proteins were up-regulated, and 1 protein was down-regulated. Additionally, 3 of the 13 proteins encoded by VNN2 (log2 fold change = 0.74; P = 0.0093), AKAP4 (log2 fold change = −0.50; P = 0.058), and DCNL4 (log2 fold change = 0.42; P = 0.079) were also differentially expressed among survivors with severe cardiomyopathy in the validation sample. No metabolites were significantly differentially expressed (FDR < 0.25) between survivors with and those without subclinical cardiomyopathy (Supplemental Figure 2).
Following the differential expression analyses of subclinical cardiomyopathy in the discovery sample, CLR-Lasso was used to identify the most informative sets of proteins and metabolites and build models to discriminate the risk for subclinical cardiomyopathy. Among the 5 models, those based on the top 20% and 25% differentially expressed proteins discriminated the risk for subclinical cardiomyopathy with the highest discrimination accuracy of 98.7% (95% CI: 94.4%-100.0%) in the discovery sample (Supplemental Table 4). Both models resulted in the same model, with the highest number of predictors selected by CLR-Lasso (n = 27). All 5 models based on the top metabolites provided discrimination accuracies less than those based on the top proteins. The highest discrimination accuracy among the metabolite-based models was 80.0% (95% CI: 63.6%-94.7%), achieved on the basis of 11 metabolites selected by CLR-Lasso among the top 20% of metabolites. Thus, the model including the 27 proteins alone was considered the best performing model for validation (Figure 1). The combination of these 27 proteins yielded a discrimination accuracy of 82.6 (95% CI: 71.4%-93.8%) for discriminating the risk for severe cardiomyopathy in the validation sample (Figure 2). The model with 11 metabolites that provided the highest discrimination accuracy in the discovery sample provided a discrimination accuracy of only 34.8% (95% CI: 20.0%-50.0%) in the validation sample.
Figure 1.
Differentially Expressed Serum Proteins in Subclinical Cardiomyopathy in Childhood Cancer Survivors Previously Treated With Anthracyclines
The x-axis shows the log2 fold change, estimating differential expression of 867 proteins quantified by tandem mass tag–based mass spectrometry between asymptomatic survivors with and without subclinical cardiomyopathy in the discovery sample, and statistical significance is shown on the y-axis. These results were obtained from a linear mixed-effects model in the discovery sample adjusted for age at cancer diagnosis, sex, cumulative anthracycline dose, race, and sample age as fixed effects and matched-pair indicator as a random effect. On the basis of these results, conditional logistic regression with a least absolute shrinkage and selection operator penalty (CLR-Lasso) was used to identify the most informative proteins and build models to discriminate the risk for subclinical cardiomyopathy. The best performing model was based on the top 20% differentially expression proteins (shown as light green triangles) and of these, CLR-Lasso selected 27 proteins (shown as red triangles).
Figure 2.
Discrimination Accuracy of the Risk Discrimination Model Based on 27 Proteins Selected by Conditional Logistic Regression With a Least Absolute Shrinkage and Selection Operator Penalty
The y-axis shows the logarithm of ratio of conditional probabilities of survivors with and without severe cardiomyopathy within each of the 23 individually matched pairs (x-axis) in the validation sample. A ratio of >1 (denoted by the dashed red horizontal line) indicates higher predicted conditional probability of a survivor with severe cardiomyopathy compared with the matched survivor without cardiomyopathy. Within each matched pair, a survivor having higher predicted conditional probability was labeled as affected, and the other matched survivor was labeled as unaffected. Discrimination accuracy was assessed by the concordance between predicted and observed cardiomyopathy outcomes per matched pair. Concordant matched pairs are shown in light green, and red shows discordant pairs.
Weights of the 27 proteins in the discovery sample’s risk discrimination equation are provided in Table 2, along with their respective functions. Eighteen of the 27 proteins had positive weights (and contributed to increased risks for subclinical cardiomyopathy), and the remaining 9 had negative weights (and contributed to decreased risk for subclinical cardiomyopathy). Six of the 27 proteins were also significantly differentially expressed (FDR <0.25) in subclinical cardiomyopathy (Table 2, Supplemental Table 3). A further 15 proteins showed differential expression by subclinical cardiomyopathy status at nominal statistical significance (P < 0.05), and the remaining 6 proteins showed P values <0.10.
Table 2.
Serum Proteins (n = 27) in the Best Performing Model From the Discovery Sample
| Protein Predictor (Accession) | Regression Coefficient (β) | Corresponding Gene(s) | Protein Names | Functions | Categories |
|---|---|---|---|---|---|
| Q15485 | 0.050 | FCN2 | Ficolin-2 (37-kDa elastin-binding protein) | Function in innate immunity through activation of the lectin complement pathway | Immune system and antigen recognition |
| Q9Y6Z7 | 0.158 | COLEC10 | Collectin-10 (collectin liver protein 1) | Acts as a chemoattractant, probably involved in the regulation of cell migration | Immune system and antigen recognition |
| O75019 | 0.023 | LIRA1 | Leukocyte immunoglobulin-like receptor A1 | Acts as a receptor for class I MHC antigens | Immune system and antigen recognition |
| A0A075B6W8 | 0.011 | TRAJ17 | T cell receptor alpha joining 17 | Bound to MHC molecules | Immune system and antigen recognition |
| A0A0F7TD49 | −0.080 | IGHV3-7 | IGHV3-7 protein | Participates in antigen recognition | Immune System and Antigen Recognition |
| P01766 | −0.060 | IGHV3-13 | Immunoglobulin heavy variable 3-13 | Participates in antigen recognition | Immune system and antigen recognition |
| Q8N6C8 | 0.012 | LILRA3 | Leukocyte immunoglobulin-like receptor A3 | May act as a soluble receptor for class I MHC antigens | Immune system and antigen recognition |
| P42765a | 0.150 | ACAA2 | 3-ketoacyl-CoA thiolase, mitochondrial | Hydrolase activity on various fatty acyl-CoAs | Metabolic enzymes |
| P07195a | 0.048 | LDHB | L-lactate dehydrogenase B chain | Interconverts pyruvate and lactate with concomitant interconversion of NADH and NAD+ | Metabolic enzymes |
| Q8WYK0a | 0.122 | ACOT12 | Acetyl-CoA thioesterase | Catalyzes the hydrolysis of acyl-CoAs into free fatty acids and CoA (CoASH) | Metabolic enzymes |
| Q96PD5 | −0.029 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | May play a scavenger role by digesting biologically active PGN into inactive fragments | Metabolic enzymes |
| Q8IZJ1 | 0.165 | UNC5B | Netrin receptor UNC5B | Receptor for netrin required for axon guidance | Neuronal functions |
| P05067 | 0.043 | APP | Amyloid-beta precursor protein | Performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion, and axonogenesis | Neuronal functions |
| Q5JQC9b | −0.475 | AKAP4 | A-kinase anchor protein 4 | Major structural component of sperm fibrous sheath | Cell structure and adhesion |
| Q2M329 | 0.180 | CFAP184 | Cilia- and flagella-associated protein 184 | Involved in cilium assembly | Cell structure and adhesion |
| Q12955 | 0.053 | ANK3 | Ankyrin-3 | Membrane-cytoskeleton linker; participates in maintenance/targeting of ion channels and cell adhesion molecules | Cell structure and adhesion |
| P68366 | −0.302 | TBA4A | Tubulin alpha-4A chain | Major constituent of microtubules | Cell structure and adhesion |
| Q9Y4G6 | 0.192 | TLN2 | Talin-2 | Involved in cytoskeletal dynamics and cellular adhesion processes | Cell structure and adhesion |
| Q5SYB0 | 0.099 | FRMPD1 | FERM and PDZ domain-containing protein 1 | Stabilizes membrane-bound GPSM1 and thereby promotes its interaction with GNAI1 | Signal transduction and regulation |
| Q8IX01 | −0.144 | SUGP2 | SURP and G-patch domain-containing protein 2 | Plays a role in mRNA splicing | Signal transduction and regulation |
| O75533a | 0.468 | SF3B1 | Splicing factor 3B subunit 1 | Involved in pre-mRNA splicing as a component of the splicing factor SF3B complex | Signal transduction and regulation |
| P28482 | −0.089 | MAPK1 | MAP kinase 1 | Serine/threonine kinase, essential in the MAP kinase signal transduction pathway | Signal transduction and regulation |
| Q9BXW9 | 0.007 | FANCD2 | Fanconi anemia group D2 protein | Required for the repair of DNA interstrand cross-links | Chromosomal stability and repair |
| Q9NVX7 | −0.005 | KBTBD4 | Kelch repeat and BTB domain-containing protein 4 | Involved in protein ubiquitination and degradation | Other functions |
| P07225 | −0.042 | PROS1 | Vitamin K–dependent protein S | Anticoagulant plasma protein | Other functions |
| Q4UJ75 | 0.171 | ANKRD20A4P | Putative ankyrin repeat domain-containing protein 20A4 | Clear function unknown | Other functions |
| Q8N7X0a | 0.017 | ADGB | Androglobin | Required for sperm flagellum formation and maturation of elongating spermatids | Other functions |
CoA = coenzyme A; MAP = mitogen-activated protein; MHC = major histocompatibility complex; mRNA = messenger RNA; NAD+ = nicotinamide-adenine dinucleotide; NADH = nicotinamide-adenine dinucleotide reduced; PGN = peptidoglycan.
Differentially expressed in the discovery sample.
Differentially expressed in both the discovery and validation samples.
Supplemental Figures 3a and 3b demonstrate that the linearity assumption of the linear mixed-effects model was approximately valid for all 27 proteins analyzed. Additionally, we did not observe any systematic trends between the fitted values and the residuals are normally distributed (Supplemental Figure 4). Supplemental Figure 5 shows the boxplot of absolute values of log predicted ORs of the concordant and discordant pairs, respectively, in the validation sample. The medians and lower and upper quantiles were 2.05 (1.11, 2.98) of |log(OR)| for concordant pairs and 1.71 (0.907, 2.86) for discordant pairs, suggesting a slight pattern consistent with good calibration.
GO enrichment analysis on the basis of the 29 proteins (27 selected by CLR-Lasso in the best performing model from the discovery sample and 3 differentially expressed in both subclinical and severe cardiomyopathy) revealed enrichments of multiple GO terms, including acetyl–coenzyme A (CoA) hydrolase activity (GO:0003986), acyl-CoA hydrolase activity (GO:0016289), fatty acetyl-CoA hydrolase activity (GO:0047617), membrane raft (GO:0045121), membrane microdomain (GO:0098857), serine-type peptidase complex (GO:1905286), and serine-type endopeptidase complexes (GO:1905370) (Supplemental Figure 6). We also identified 7 PPIs among these proteins. When we expanded our analysis to include all 867 proteins, we discovered that 12 of these proteins interacted with at least 10 partner proteins through physical and functional interactions.
Discussion
Using a well-characterized cohort of long-term survivors of childhood cancer with clinically assessed anthracycline-associated cardiomyopathy and serum profiling of proteins using an untargeted mass spectrometry–based approach, we developed a model to discriminate the risk for severe cardiomyopathy requiring heart failure therapy (Central Illustration). The model included 27 proteins that were dysregulated in asymptomatic survivors with subclinical cardiomyopathy and provided discrimination accuracy of 83% in an independent sample, indicating potential for clinical utility. These proteins also highlighted important biological processes involved in maintaining cardiac function and contributing to heart-related diseases.
Central Illustration.
Serum Proteins Discriminating Cardiomyopathy Risk in Childhood Cancer Survivors Exposed to Anthracyclines Without Chest Radiation
The study identified a combination of 27 serum proteins that accurately discriminated the risk for severe cardiomyopathy with 83% accuracy among survivors of childhood cancer previously exposed to anthracyclines but not chest radiation.
Cardiotoxic late effects of anthracyclines generally manifest as decreased left ventricular function, often with irreversible progression to heart failure. Thus, early detection of cardiac dysfunction may allow early intervention with preventive strategies. To this end, we developed a model among asymptomatic survivors with and without subclinical cardiomyopathy and evaluated its ability to discriminate severe cardiomyopathy risk. The model included serum proteins dysregulated in subclinical cardiomyopathy, possibly indicating subtle changes before clinically overt signs and symptoms of heart failure. Therefore, if further validated in prospective studies, our model may be useful in individual risk prediction of severe cardiomyopathy among asymptomatic survivors or those with early-stage cardiac dysfunction, thereby providing opportunities for preventive interventions.
In the general population, circulating proteins have been shown to improve individual risk prediction of cardiovascular diseases beyond the currently available tools. A model based on 50 circulating proteins measured using a proximity extension assay outperformed (change in area under the curve [AUC]: 0.10) the clinical risk model in predicting the atherosclerotic cardiovascular events in 2 primary prevention populations.28 Another study used the same method to measure 276 proteins in 2 secondary prevention cohorts and found that a 50-protein model significantly improved (change in AUC: 0.04) the risk prediction of major adverse cardiovascular events on the basis of clinical parameters alone in the validation cohort.29 More recently, Williams et al30 measured 5,000 proteins using the aptamer-based technique and found that a 27-protein model was superior (change in AUC: 0.06) than a clinical model in predicting the risk for major adverse cardiovascular events in both primary and secondary prevention cohorts. In childhood cancer survivors, studies assessing circulating proteins as predictors of cardiac dysfunction have predominantly used immunoassays, which rely on predesigned markers and offer limited throughput.16,31,32 Leerink et al15 used the proximity extension assay to measure 276 plasma proteins and developed a prediction model that discriminated between childhood cancer survivors with and without cardiomyopathy with an AUC of 0.78. However, that model was not validated in an independent sample, and the proteins were a priori selected on the basis of their associations with cardiovascular disease using Olink Proteomics,15 limiting the ability to identify previously unknown biomarkers. To our knowledge, our study is the first to use an untargeted mass spectrometry–based approach to identify novel serum proteins and develop and independently validate a model to discriminate the risk for severe cardiomyopathy among long-term survivors of childhood cancer.
Identified novel proteins that were discriminatory of cardiomyopathy highlighted potential pathophysiological mechanisms underlying anthracycline-related cardiomyopathy in childhood cancer survivors. One such protein is encoded by AKAP4, which belongs to the AKAP family of scaffolding proteins known for their roles in cardiac health and disease. For instance, AKAP13 fosters hypertrophy and fibrosis,33 and AKAP150 influences heart dynamics through calcium modulation, ion activity, and protein kinase C stimulation.34 Eliminating AKAP13 in mice resulted in flawed cardiac development.35 Deleting AKAP1 prompted cardiac cell recycling and cell death postinjury, indicating its involvement in heart cell energy processes.36 In addition, knockout studies in animal models have shown that genetic variants in AKAPs increase the risk for cardiovascular diseases, including heart rhythm abnormalities, heart failure, and sudden cardiac death.37 SF3B1 has been linked to cardiac hypertrophy in hypoxic conditions.38,39 Recently, a higher prevalence of SF3B1-mutated clonal hematopoiesis of intermediate potential (CHIP) was identified among patients with heart failure.40 Moreover, patients with SF3B1-mutated CHIP exhibited elevated levels of ferritin compared with patients with non-SF3B1-mutated CHIP and those without CHIP, indicating disrupted iron homeostasis as a potential etiology for heart failure. Tubulins have been linked to many heart diseases, such as ischemic, hypertrophic, and dilated cardiomyopathies and heart failure.41 TBA4A encodes tubulin alpha 4a and represents a major component of microtubules. In failing cardiomyocytes, microtubule networks were found to be dense and highly detyrosinated, resulting in enhanced cardiomyocyte stiffness and decreased contractility.42 Pharmacologic or genetic suppression of microtubule detyrosination could recover 40% to 50% of lost contractile performance.42 Similarly, hyperacetylation of tubulin has been reported in cardiomyopathy and heart failure in mouse models.43 The inhibition of tubulin deacetylation using a histone deacetylase inhibitor was found to improve cardiac function.43
Study limitations
Our sample size was small. To address potential confounding, we used a matched-pair study design in which survivors without cardiomyopathy were individually matched to those with cardiomyopathy on the basis of known risk factors. Because of this matched design and validation of our findings in an independent sample, our results were less likely to be influenced by these factors and are thus robust. However, as survivors in both the discovery and validation samples were participants from SJLIFE, external validation in a larger cohort is warranted to further validate our findings and evaluate their clinical utility for early detection of anthracycline-induced cardiomyopathy.
We also minimized potential experimental bias by using a single-blinded approach to randomize all matched pairs into 14 batches for mass spectrometry–based proteomics and metabolomics. Serum samples from fewer than one-half of the survivors in this study were obtained during an earlier SJLIFE visit, leading to a period of <5 years between serum collection and cardiomyopathy evaluation. As a result, some of the proteins identified might reflect physiological processes that makes one susceptible to cardiomyopathy or perturbations related to early remodeling or injury signatures of progressive cardiomyopathy rather than being indicative of late effects from childhood anthracycline exposure. Future research can help inform this by using biospecimens collected nearer to the time of cardiomyopathy assessment. Serum metabolites were not found to be differentially expressed in, or discriminative of, cardiomyopathy, which may be due partly to our small sample size. Future studies in a larger sample of survivors are needed to investigate the role of circulating metabolites in treatment-related cardiac dysfunction. Although cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes are known risk factors of cardiomyopathy, they could not be matched between survivors with and without cardiomyopathy because of sample availability limitation. Furthermore, the combination of 27 proteins perfectly discriminated survivors with and without subclinical cardiomyopathy in the discovery sample, and we were thus unable to evaluate the additional covariates including cardiovascular risk factors.
Conclusions
We identified a combination of 27 serum proteins that accurately discriminated the risk for severe cardiomyopathy in childhood cancer survivors previously treated with anthracyclines without chest irradiation. Therefore, our protein-based validated model needs to be further evaluated in an external larger cohort and prospective studies, as it may be clinically useful for early detection of subclinical cardiac dysfunction, thereby providing opportunities for lifestyle interventions to delay or prevent the onset of symptomatic and severe cardiomyopathy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Anthracyclines, a highly effective chemotherapy for many pediatric malignancies, cause cardiomyopathy, a clinically important late effect in adult survivors. Biomarkers could inform the early detection and targeted interventions for cardiomyopathy. Using an untargeted mass spectrometry–based approach, we profiled 867 proteins and 218 metabolites in serum samples from 75 asymptomatic survivors with subclinical cardiomyopathy and 75 individually matched survivors without cardiomyopathy from SJLIFE. A 27-protein model identified by CLR-Lasso accurately discriminated symptomatic or severe cardiomyopathy requiring heart failure medications in an independent sample of SJLIFE survivors; 19 of 23 individually matched survivors with and without cardiomyopathy were correctly discriminated with 83% accuracy.
TRANSLATIONAL OUTLOOK: Circulating proteins have potential clinical utility in screening and detecting early-stage cardiac dysfunction, thereby providing opportunities for interventions to delay or prevent the onset of symptomatic and severe cardiomyopathy.
Funding Support and Author Disclosures
This study was supported by the National Cancer Institute of the National Institutes of Health (grant R01 CA261898, Drs Burridge and Sapkota, principal investigators; grant R01 CA216354 and Dr Yasui, principal investigator; grant R21 CA261833 and Dr Im, principal investigator; grant U24 CA55727, Dr Armstrong, principal investigator; grant U01 CA195547, Drs Hudson and Ness, principal investigators; and Cancer Center Support [CORE] grant CA21765, C. Roberts, principal investigator) and the American Lebanese Syrian Associated Charities. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Eric Chow, MD, MPH, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For information on protein and metabolite measurements as well as supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Hudson M.M., Ness K.K., Gurney J.G., et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dalen E.C., Raphael M.F., Caron H.N., Kremer L.C. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev. 2014;(9) doi: 10.1002/14651858.CD006647.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulrooney D.A., Armstrong G.T., Huang S., et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney D.A., Hyun G., Ness K.K., et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368 doi: 10.1136/bmj.l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker G.M., Thompson R.E., Hare J.M., et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 6.Armenian S.H., Hudson M.M., Mulder R.L., et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt M.J., Leerink J.M., Mulder R.L., et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2023;24:e108–e120. doi: 10.1016/S1470-2045(23)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Shankar S.M., Marina N., Hudson M.M., et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz S.E., Lipsitz S.R., Sallan S.E., et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–4522. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 10.Leerink J.M., Verkleij S.J., Feijen E.A.M., et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart. 2019;105:210–216. doi: 10.1136/heartjnl-2018-313634. [DOI] [PubMed] [Google Scholar]
- 11.Pourier M.S., Kapusta L., van Gennip A., et al. Values of high sensitive troponin T in long-term survivors of childhood cancer treated with anthracyclines. Clin Chim Acta. 2015;441:29–32. doi: 10.1016/j.cca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Mavinkurve-Groothuis A.M., Groot-Loonen J., Bellersen L., et al. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatr Blood Cancer. 2009;52:631–636. doi: 10.1002/pbc.21913. [DOI] [PubMed] [Google Scholar]
- 13.Dixon S.B., Howell C.R., Lu L., et al. Cardiac biomarkers and association with subsequent cardiomyopathy and mortality among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort. Cancer. 2021;127:458–466. doi: 10.1002/cncr.33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrhardt M.J., Liu Q., Mulrooney D.A., et al. Improved cardiomyopathy risk prediction using global longitudinal strain and N-terminal-pro-B-type natriuretic peptide in survivors of childhood cancer exposed to cardiotoxic therapy. J Clin Oncol. 2024;42(11):1265–1277. doi: 10.1200/JCO.23.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leerink J.M., Feijen E.A.M., Moerland P.D., et al. Candidate plasma biomarkers to detect anthracycline-related cardiomyopathy in childhood cancer survivors: a case control study in the Dutch Childhood Cancer Survivor Study. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.025935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armenian S.H., Gelehrter S.K., Vase T., et al. Carnitine and cardiac dysfunction in childhood cancer survivors treated with anthracyclines. Cancer Epidemiol Biomarkers Prev. 2014;23:1109–1114. doi: 10.1158/1055-9965.EPI-13-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell C.R., Bjornard K.L., Ness K.K., et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50:39–49. doi: 10.1093/ije/dyaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson M.M., Ness K.K., Nolan V.G., et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson M.M., Ehrhardt M.J., Bhakta N., et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feijen E.A.M., Leisenring W.M., Stratton K.L., et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates D., Machler M., Bolker B.M., Walker S.C. Fitting Linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 22.Benjamini Y., Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 23.Reid S., Tibshirani R. Regularization paths for conditional logistic regression: the clogitL1 package. J Stat Softw. 2014;58:1–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Reimand J., Arak T., Adler P., et al. g:Profiler—a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon P., Markiel A., Ozier O., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doncheva N.T., Morris J.H., Gorodkin J., Jensen L.J. Cytoscape StringApp: network analysis and visualization of proteomics data. J Proteome Res. 2019;18:623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P., Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogeveen R.M., Pereira J.P.B., Nurmohamed N.S., et al. Improved cardiovascular risk prediction using targeted plasma proteomics in primary prevention. Eur Heart J. 2020;41:3998–4007. doi: 10.1093/eurheartj/ehaa648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurmohamed N.S., Belo Pereira J.P., Hoogeveen R.M., et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur Heart J. 2022;43:1569–1577. doi: 10.1093/eurheartj/ehac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams S.A., Ostroff R., Hinterberg M.A., et al. A proteomic surrogate for cardiovascular outcomes that is sensitive to multiple mechanisms of change in risk. Sci Transl Med. 2022;14(639) doi: 10.1126/scitranslmed.abj9625. [DOI] [PubMed] [Google Scholar]
- 31.Armenian S.H., Gelehrter S.K., Vase T., et al. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res. 2014;20:6314–6323. doi: 10.1158/1078-0432.CCR-13-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ky B., Putt M., Sawaya H., et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Vescovo C.D., Cotecchia S., Diviani D. A-kinase-anchoring protein-Lbc anchors IkappaB kinase beta to support interleukin-6-mediated cardiomyocyte hypertrophy. Mol Cell Biol. 2013;33:14–27. doi: 10.1128/MCB.00887-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L., Li J., Drum B.M., et al. Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve. Cardiovasc Res. 2017;113:147–159. doi: 10.1093/cvr/cvw221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayers C.M., Wadell J., McLean K., et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiattarella G.G., Cattaneo F., Pironti G., et al. Akap1 deficiency promotes mitochondrial aberrations and exacerbates cardiac injury following permanent coronary ligation via enhanced mitophagy and apoptosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suryavanshi S.V., Jadhav S.M., McConnell B.K. Polymorphisms/mutations in A-kinase anchoring proteins (AKAPs): role in the cardiovascular system. J Cardiovasc Dev Dis. 2018;5(1):7. doi: 10.3390/jcdd5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirtschink P., Krishnan J., Grimm F., et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature. 2015;522:444–449. doi: 10.1038/nature14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W., Yang J., Zhang D., et al. Role of Bcl-2/adenovirus E1B 19 kDa-interacting protein 3 in myocardial cells in diabetes. Exp Ther Med. 2015;10:67–73. doi: 10.3892/etm.2015.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas T., Ji Y.Y., Kalkan F., et al. Clonal hematopoiesis and heart failure with preserved ejection fraction. Blood. 2022;140:8611–8612. [Google Scholar]
- 41.Liu C., Chen Y., Xie Y., Xiang M. Tubulin post-translational modifications: potential therapeutic approaches to heart failure. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.872058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C.Y., Caporizzo M.A., Bedi K., et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med. 2018;24:1225–1233. doi: 10.1038/s41591-018-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLendon P.M., Ferguson B.S., Osinska H., et al. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc Natl Acad Sci U S A. 2014;111:E5178–E5186. doi: 10.1073/pnas.1415589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.