Abstract
Background
Patients with lymphoma are at high risk for developing heart failure (HF) after autologous hematopoietic cell transplantation (HCT). More accurate risk determination pre-HCT may facilitate screening and prevention of HF.
Objectives
The aim of this study was to examine the association between clonal hematopoiesis of indeterminate potential (CHIP) and the risk for HF after HCT for lymphoma.
Methods
This was a retrospective cohort study of 861 patients who underwent autologous HCT for lymphoma between 2010 and 2016 at City of Hope Comprehensive Cancer Center. Targeted DNA sequencing was performed to determine the presence of CHIP (variant allele frequency ≥ 2%). The primary outcome of interest was the 5-year cumulative incidence of de novo HF. Other outcomes of interest included overall and cause-specific mortality.
Results
Overall, 186 patients (21.7% of the cohort) had at least 1 CHIP variant, and 59 (6.9%) had ≥2 variants. DNMT3A, PPM1D, and TET2 were the most frequently mutated genes. The 5-year incidence of HF was significantly higher in patients with CHIP compared with those without CHIP (13.8% vs 4.7%; P < 0.001; sub-distribution hazard ratio [sHR]: 2.48; 95% CI: 1.32-4.68); the HF incidence increased by variant allele frequency: 0-2% (4.7%), 2-10% (11.7%), and >10% (18.5%), P < 0.001. Patients with CHIP had significantly worse overall survival after HCT, compared with those without (63.4% vs 80.3%; P < 0.001), due primarily to the higher risk for nonrelapse mortality (subdistribution HR: 5.37; 95% CI: 2.34-12.35).
Conclusions
CHIP was highly prevalent and associated with risk for HF and nonrelapse mortality after HCT. These findings highlight the role of CHIP as a novel biomarker and potential target for intervention to improve outcomes after autologous HCT.
Key Words: autologous hematopoietic cell transplantation, biomarkers, CHIP, heart failure, lymphoma, nonrelapse mortality
Central Illustration
Autologous hematopoietic cell transplantation (HCT) is an established therapeutic option for patients with relapsed or refractory lymphoma.1, 2, 3, 4 However, HCT survivors are at risk for developing life-threatening chronic health conditions that contribute to a higher burden of nonrelapse mortality (NRM) compared with the general population.5, 6, 7, 8 Heart failure (HF) is a well-recognized complication after HCT, attributed to the cumulative effects of cardiotoxic therapies (eg, anthracycline chemotherapy, chest radiation therapy [RT]) and modifiable cardiovascular risk factors (eg, hypertension, diabetes).9,10 Five-year survival rates after the onset of HF in this population are <50%,9,11 emphasizing the need to refine HF risk determination prior to HCT, which would allow consideration of therapeutic alternatives or the implementation of primary prevention after HCT.
Clonal hematopoiesis of indeterminate potential (CHIP) involves the clonal expansion of hematopoietic stem cells driven by somatic mutations in leukemogenic genes in the absence of leukemia.12 CHIP has been recognized as an aging phenomenon and linked to increased risk for a wide range of cardiovascular diseases, including cardiomyopathy and HF.13, 14, 15, 16 Accumulating data suggest that there is a higher burden of CHIP among patients with cancer compared with the general population, likely driven by therapeutic exposures, chronic stress, and inflammation.17, 18, 19, 20 However, whether CHIP contributes to the higher risk for HF after HCT for lymphoma is unknown. Therefore, we examined the association between pre-HCT CHIP and HF in a demographically diverse cohort of patients with Hodgkin and non-Hodgkin lymphoma undergoing HCT. Additionally, we explored the relationship between modifiable cardiovascular risk factors and CHIP in moderating HF risk and the impact of CHIP on survival after HCT.
Methods
Study population and clinical variables
This study included a retrospective cohort of patients with lymphoma who underwent first autologous HCT at City of Hope Comprehensive Cancer Center between 2010 and 2016 and had mobilized peripheral blood stem cell (PBSC) products cryopreserved and accessible for CHIP analyses (861 of 867 eligible patients [99.3%]). Information related to patient demographics (eg, age at HCT, sex, race/ethnicity), diagnosis (eg, lymphoma subtypes, pre-HCT treatment), variables needed to calculate the HCT-specific comorbidity index, and HCT details (eg, conditioning, PBSC mobilization regimen, CD34+ cell count) were abstracted from medical records.21 The City of Hope Institutional Review Board reviewed and approved the study (#18076) and granted a waiver of the requirement to obtain informed consent and Health Insurance Portability and Accountability Act authorization.
Health conditions associated with risk for cardiovascular disease, including hypertension, diabetes, and dyslipidemia, were captured if they were documented by treating physicians and if patients were receiving medications for their management at HCT. A high HCT-specific comorbidity index was defined as ≥3.22 Patients were considered in complete remission per established guidelines.23 Incident HF was defined as 1) new diagnosis of HF or related diagnosis (eg, left ventricular dysfunction); and 2) new left ventricular ejection fraction (EF) decrease to <50% by echocardiography and/or clinical evidence of HF (eg, dyspnea on exertion, lower extremity edema).24 The following protocol was implemented to ensure adequate and thorough follow-up after HCT. If the most recent medical visit at City of Hope was outdated or there were gaps in a patient’s history during the 5-year follow-up period after HCT, a standard protocol was used to identify and contact physicians treating patients external to City of Hope to gather pertinent health details. If the physician was unavailable or unable to provide relevant information, the patient was directly contacted to obtain the information. Vital status and cause-of-death information was obtained from the National Death Index and medical records. Relapse-related mortality included death due to the primary disease or lymphoma. NRM included death due to all other causes.
Next-generation sequencing and CHIP variant calling
DNA was extracted from cryopreserved mobilized PBSC samples using the QIAamp DNA Mini Kit (Qiagen). Targeted panel-based DNA sequencing of the isolated DNA was carried out using a QIAseq amplicon-based panel consisting of 108 CHIP-associated genes (Qiagen) (Supplemental Table 1). The DNA library preparation including polymerase chain reaction multiplex amplification was performed by the City of Hope Division of Clinical Cancer Genomics laboratory. Library quality and size distribution were assessed using the Agilent 2100 Bioanalyzer system. Pair-end sequencing with a coverage depth of 1,000× (PE151) was performed at the City of Hope Integrative Genomics Core on a NovaSeq S4 flow cell (Illumina), achieving an average target coverage read depth of 560×. Overall, 99.8% (1,395 of 1,398) of the targets were covered over 1,000× read depth.
Raw sequence reads were aligned to the human genome (GRCh37/hg19), and variant calling and annotation were independently conducted using 2 software applications: CLC Genomics Workbench (CLCBio) and NextGENE (SoftGenetics). We followed established guidelines (Catalog of Somatic Mutations in Cancer) for variant classification and CHIP calling.12,25 CHIP was defined with variant allele frequency (VAF) ≥2% on the basis of established guidelines.12
Statistical analysis
Continuous data are presented as median (range) and categorial data as count (percentage). We conducted univariable analyses to compare patient demographics, comorbidity burden (HCT-specific comorbidity index, cardiovascular risk factors), pre-HCT history of another malignancy, lymphoma characteristics (histology, remission status at HCT), pre-HCT treatment (eg, chemotherapy, chest RT), and HCT-related variables (conditioning regimen, PBSC mobilization, PBSC CD34+ cell count) between patients with and without CHIP at HCT. Categorical variables were compared using 2-sided chi-square tests, while continuous variables were analyzed using 2-sample Wilcoxon rank sum tests (medians) for non-normally distributed data. Multivariable logistic regression was used to characterize variables associated with risk (OR) of having CHIP. The regression model included variables with P values <0.10 in the univariable analyses.
Because of the known latency of HF (median 2-3 years),9,26 follow-up was extended to include up to 5 years post-HCT. The time to the first HF event was calculated from the HCT date to HF onset, last known alive date, receipt of second HCT, date of death, or 5 years after HCT, whichever came first. All-cause mortality was considered as a competing risk. Patients surviving >5 years after HCT were censored at 5 years. Those with histories of HF before HCT (n = 34) were excluded from the analysis. Additionally, exploratory analyses were conducted to assess the association between HF incidence and CHIP variables, including: 1) specific CHIP genes; 2) the number of CHIP variants (no CHIP vs 1 CHIP vs ≥2 CHIP variants); 3) VAF categories (no CHIP vs VAF 2% to ≤10% vs VAF > 10%). For patients with multiple CHIP variants, we categorized VAF on the basis of the highest observed value.
We conducted univariable and multivariable analyses using the Fine-Gray subdistribution hazard model, calculating subdistribution HRs (sHRs) and their 95% CIs to quantify the magnitude of the risk for developing HF. To construct the multivariable model, we first examined the association between baseline clinical variables at HCT and the cumulative incidence of HF by performing univariable analysis. Variables with P values <0.10 in the univariable analysis were then included, and backward stepwise elimination was used to construct the final multivariable model.
To examine the interplay among individual modifiable pre-HCT cardiovascular risk factors (hypertension, diabetes, dyslipidemia), CHIP, and the subsequent risk for HF, we constructed separate models to assess the risk for HF for individuals in the following categories: 1) no pre-HCT cardiovascular risk factors and no CHIP (the referent group); 2) pre-HCT cardiovascular risk factors but no CHIP; 3) no pre-HCT cardiovascular risk factors but with CHIP; and 4) both cardiovascular risk factors and CHIP. We also examined whether there was a statistically significant interaction between individual cardiovascular risk factors and CHIP in moderating HF risk. The cumulative incidence of HF within each category was computed and compared using Gray’s competing risk method, and multivariable regression analyses (Fine-Gray model) were conducted to account for potential confounding.
The Kaplan-Meier method was used to examine the impact of CHIP on all-cause mortality; log-rank tests were used to compare survival curves. Multivariable Cox regression analysis was used to examine the association between CHIP and all-cause mortality, and Fine-Gray regression analysis was used to examine the association between CHIP and NRM as well as relapse-related mortality, with each outcome serving as competing risk. To verify the proportional hazards assumption in a Cox model for all-cause mortality, we tested the interactions between each covariate in the multivariable model and time, and any significant interactions were then included in the model. All analyses were performed using SAS version 9.4 (SAS Institute). All statistical analyses were 2 sided, and a P value <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The demographic and clinical characteristics of the overall cohort (n = 861) are included in Table 1. The median age at HCT was 55.7 years (range: 18.4-78.1 years), and the majority were men (63.3%), had a high (≥3) HCT-specific comorbidity index (46.1%), and were diagnosed with non-Hodgkin lymphoma (78.0%). The racial and ethnic distribution was as follows: 56.9% non-Hispanic White, 26.1% Hispanic, 10.8% Asian, and 6.2% Black or other. The vast majority (94.3%) had received anthracycline-based therapy for their lymphoma (median dose 300 mg/m2), and 5.9% had received chest RT. PBSC mobilization was with granulocyte colony-stimulating factor alone or granulocyte colony-stimulating factor and cyclophosphamide in 64.4%, and carmustine, etoposide, cytarabine, and melphalan was the most common conditioning regimen. The demographic and clinical characteristics of patients without histories of HF (n = 827) for subsequent outcome analyses are included in Supplemental Table 2.
Table 1.
Demographic and Clinical Characteristics of Patients With HCT
| Total (N = 861) | CHIP (n = 186) | No CHIP (n = 675) | P Value | |
|---|---|---|---|---|
| Age at HCT, y | 55.7 (18.4-78.1) | 64.6 (34.2-77.3) | 51.2 (18.4-78.1) | <0.001 |
| Sex | ||||
| Female | 316 (36.7) | 68 (36.6) | 248 (36.7) | 0.96 |
| Male | 545 (63.3) | 118 (63.4) | 427 (63.3) | |
| Race/ethnicity | ||||
| Asian | 93 (10.8) | 24 (12.9) | 69 (10.2) | 0.024 |
| Hispanic | 225 (26.1) | 36 (19.4) | 189 (28.0) | |
| Non-Hispanic White | 490 (56.9) | 119 (64.0) | 371 (55.0) | |
| Black/other | 53 (6.2) | 7 (3.8) | 46 (6.8) | |
| BMI, kg/m2 | 27.8 (15.9-51.4) | 27.1 (18.3-42.5) | 27.9 (15.9-51.4) | 0.045 |
| HCT-specific comorbidity index | ||||
| 0 | 202 (23.5) | 35 (18.8) | 167 (24.7) | 0.24 |
| 1 or 2 | 262 (30.4) | 60 (32.3) | 202 (29.9) | |
| ≥3 | 397 (46.1) | 91 (48.9) | 306 (45.3) | |
| Diagnosis | ||||
| Hodgkin lymphoma | 189 (22.0) | 8 (4.3) | 181 (26.8) | <0.001 |
| Non-Hodgkin lymphoma | 672 (78.0) | 178 (95.7) | 494 (73.2) | |
| DLBCL | 369 (54.9) | 89 (50.0) | 280 (56.7) | <0.001 |
| Follicular | 53 (7.9) | 17 (9.6) | 36 (7.3) | |
| Mantle cell | 127 (18.9) | 37 (20.8) | 90 (18.2) | |
| Other | 123 (18.3) | 35 (19.7) | 88 (17.8) | |
| Pre-HCT anthracycline | 812 (94.3) | 176 (94.6) | 636 (94.2) | 0.97 |
| Pre-HCT chest radiation | 51 (5.9) | 14 (7.5) | 37 (5.5) | 0.38 |
| Remission status at HCT | ||||
| CR | 555 (64.5) | 125 (67.2) | 430 (63.7) | 0.43 |
| Not in CR | 306 (35.5) | 61 (32.8) | 245 (36.3) | |
| Conditioning regimen | ||||
| BEAM | 607 (70.5) | 159 (85.5) | 448 (66.4) | <0.001 |
| CBV | 205 (23.8) | 20 (10.8) | 185 (27.4) | |
| Other | 49 (5.7) | 7 (3.8) | 42 (6.2) | |
| PBSC mobilization regimen | ||||
| G-CSF only | 271 (31.5) | 47 (25.27) | 224 (33.2) | 0.031 |
| G-CSF + cyclophosphamide | 283 (32.9) | 56 (30.1) | 227 (33.6) | |
| G-CSF + plerixafor | 140 (16.3) | 37 (19.9) | 103 (15.3) | |
| G-CSF + cyclophosphamide + plerixafor | 167 (19.4) | 46 (24.7) | 121 (17.9) | |
| PBSC CD34+ count | ||||
| >3 × 106 cells/kg | 757 (87.9) | 160 (86.0) | 597 (88.4) | 0.44 |
| ≤3 × 106 cells/kg | 104 (12.1) | 26 (14.0) | 78 (11.6) | |
| Baseline CV risk factors | ||||
| Hypertension | 276 (32.1) | 77 (41.4) | 199 (29.5) | 0.003 |
| Diabetes | 122 (14.2) | 33 (17.7) | 89 (13.2) | 0.15 |
| Dyslipidemia | 211 (24.5) | 64 (34.4) | 147 (21.8) | 0.001 |
| Pre-HCT history of malignancy | 114 (13.2) | 45 (24.2) | 69 (10.2) | <0.001 |
Values are median (range) or n (%).
BEAM = carmustine, etoposide, cytarabine, and melphalan; BMI = body mass index (calculated from height and weight at the time of hematopoietic cell transplantation); CBV = cyclophosphamide, carmustine, and etoposide; CHIP = clonal hematopoiesis of indeterminate potential; CR = complete remission; CV = cardiovascular; DLBCL = diffuse large B cell lymphoma; G-CSF = granulocyte colony-stimulating factor; HCT = hematopoietic cell transplantation; PBSC = peripheral blood stem cell.
In the overall cohort, 186 patients (21.6% of the cohort) had at least 1 CHIP variant, and 59 (6.9%) had ≥2 variants (Figure 1A, Supplemental Table 3). The prevalence of CHIP increased with age: 4.2% for <50 years, 20.4% for 50 to 59 years, 36.8% for 60 to 69 years, and 52.2% for ≥70 years (Figure 1B). Among patients with CHIP, the calculated VAFs were 2% to 10% in 131 patients (70.4%) and >10% in 55 patients (29.6%) (Figure 1C). DNMT3A was the most frequently mutated gene (33.1%), followed by PPM1D (24.6%; majority nonsense or frameshift mutations), TET2 (14.4%), and TP53 (5.9%; majority missense mutations) (Figures 1D and 1E). The comutational pattern across genes is depicted in Figure 1F. In the multivariable model, factors independently associated with odds of having CHIP were older age (>55.7 years [median]) at HCT (OR: 5.03; 95% CI: 3.18-7.97) and a history of having another malignancy prior to the diagnosis of lymphoma (OR: 1.79; 95% CI: 1.12-2.84) (Supplemental Table 4).
Figure 1.
Characteristics of CHIP Mutations
(A) Number of patients harboring clonal hematopoiesis of indeterminate potential (CHIP) mutations in 1, 2, and 3 or more different genes. (B) Prevalence of CHIP according to age groups at transplantation. (C) Spectrum of variant allele frequencies (VAFs) in genes. (D) Number of patients with specific gene mutations. (E) Percentages of the different mutation subtypes for 4 of the most prevalent CHIP genes. Red denotes missense, green denotes nonsense, blue denotes frameshift, and yellow denotes splicing variants. (F) Comutation plot showing mutations present in all 186 patients: each column represents a single patient. Solid green denotes a single mutation, green with a white slash denotes double mutations, and solid red denotes triple mutations in the same gene. The VAF cutoff used to call mutations was 0.02. Max = maximum.
CHIP and the incidence of HF
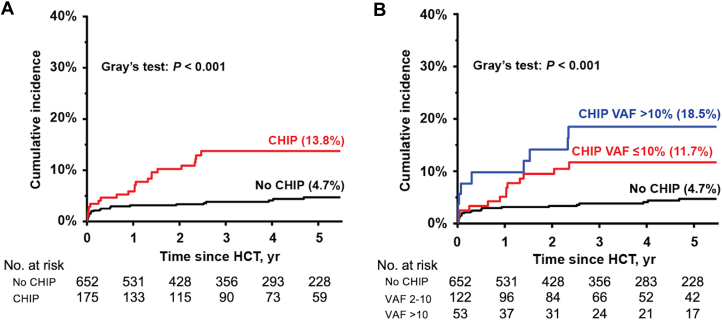
There were 48 patients who developed de novo HF within 5 years from HCT with a median time to incident HF of 187 days (range: 3-1,784 days). In a subset of patients with available EF data (n = 45), median EF was 45% (range: 18%-63%), and 18 patients (40.0%) had HF with preserved EF, defined as EF ≥50%. The 5-year cumulative incidence of HF was significantly higher among patients with CHIP compared with those without CHIP (13.8% vs 4.7%; P < 0.001) (Figure 2A). A graded relationship between VAF and the incidence of HF was observed across the following categories: no CHIP (4.7%), VAF ≤ 10% (11.7%), and VAF >10% (18.5%) (P < 0.001) (Figure 2B). Similarly, a graded relationship between the number of CHIP variants and the incidence of HF was noted by the following categories: no CHIP (4.7%), 1 CHIP variant (11.9%), and ≥2 variants (25.6%) (P < 0.001). We then explored gene-specific associations by examining the incidence of HF according to the 3 most prevalent CHIP genes (DNMT3A, PPM1D, and TET2). The most significant association was observed with TET2, for which 9 of 33 patients (27.3%) with the variant developed HF, compared with 26 of 652 patients without CHIP (4.0%) (P < 0.001) (Supplemental Table 5).
Figure 2.
Five-Year Cumulative Incidence of Heart Failure
Five-year cumulative incidence of heart failure according to (A) the presence of CHIP and (B) CHIP VAF categories (no CHIP, VAF ≤ 10%, and VAF > 10%). HCT = hematopoietic cell transplantation; other abbreviations as in Figure 1.
In univariable analysis, CHIP was associated with a >3-fold risk for HF (sHR: 3.12; 95% CI: 1.77-5.49) at 5 years. Other significant risk factors for HF included older age (>55.7 years) age (sHR: 3.01; 95% CI: 1.56-5.79), female sex (sHR: 1.91; 95% CI: 1.09-3.37), high HCT-specific comorbidity index (sHR: 2.73; 95% CI: 1.48-5.02), non-Hodgkin lymphoma diagnosis (sHR: 4.35; 95% CI: 1.36-13.97), and hypertension (sHR: 2.66; 95% CI: 1.51-4.70) (Table 2). No significant differences were observed in the risk for HF on the basis of rates of anthracycline therapy exposure or by cumulative dose categories. In the multivariable model, CHIP was significantly and independently associated with risk for HF (sHR: 2.48; 95% CI: 1.32-4.68) after HCT (Table 2). The association between CHIP and HF remained significant irrespective of: 1) censoring follow-up at second HCT; and 2) using conventional thresholds for age (<50, 50 to <60, and ≥60 years) and body mass index (<25, 25 to <30, and ≥30 kg/m2), which were selected for clinical interpretability and applicability. Next, to compare CHIP with established clinically significant risk factors for HF in patients with lymphoma, we developed a multivariable model that included CHIP, median age at HCT, gender, anthracycline dose, pre-HCT diabetes, coronary artery disease, and post-HCT therapy–related malignant neoplasm (Supplemental Table 6). CHIP remained a significant independent risk factor for post-HCT HF, with a risk comparable with that of the original model.
Table 2.
Univariable and Multivariable Analyses of Risk for Heart Failure After HCT
| Heart Failure |
||||
|---|---|---|---|---|
| Univariable Analysis |
Multivariable Analysis |
|||
| sHR (95% CI) | P Value | sHR (95% CI) | P Value | |
| CHIP | 3.12 (1.77-5.49) | <0.001 | 2.48 (1.32-4.68) | 0.005 |
| Median age (55.7 y) | ||||
| <55.7 y | 1.00 (reference) | — | ||
| ≥55.7 y | 3.01 (1.56-5.79) | 0.001 | 1.65 (0.81-3.38) | 0.17 |
| Gender | ||||
| Male | 1.00 (reference) | — | ||
| Female | 1.91 (1.09-3.37) | 0.024 | 1.74 (0.98-3.11) | 0.059 |
| Race | ||||
| Non-Hispanic White | 1.00 (reference) | — | ||
| Asian | 1.26 (0.56-2.86) | 0.58 | ||
| Hispanic | 0.73 (0.36-1.50) | 0.39 | ||
| Black/other | 0.73 (0.17-3.04) | 0.66 | ||
| BMI, kg/m2 | 0.96 (0.92-1.01) | 0.15 | ||
| HCT-specific comorbidity index | ||||
| <3 | 1.00 (reference) | — | ||
| ≥3 | 2.73 (1.48-5.02) | 0.001 | 2.30 (1.25-4.21) | 0.007 |
| Diagnosis | ||||
| HL | 1.00 (reference) | — | ||
| NHL | 4.35 (1.36-13.97) | 2.43 (0.71-8.33) | 0.16 | |
| NHL subtypes vs HLa | ||||
| DLBCL | 4.22 (1.27-13.99) | 0.019 | ||
| Follicular | 4.74 (1.07-20.89) | 0.040 | ||
| Mantle cell | 4.93 (1.36-17.79) | 0.015 | ||
| Other | 3.93 (1.02-15.16) | 0.047 | ||
| Pre-HCT anthracycline | 1.35 (0.32-5.61) | 0.68 | ||
| Pre-HCT anthracycline by category | ||||
| <150 mg/m2 | 1.00 (reference) | — | ||
| 150 to <250 mg/m2 | 1.34 (0.38-4.69) | 0.65 | ||
| 250 to <350 mg/m2 | 0.93 (0.29-3.01) | 0.91 | ||
| ≥350 mg/m2 | 1.15 (0.28-4.72) | 0.84 | ||
| Pre-HCT chest radiation | 1.42 (0.52-3.86) | 0.50 | ||
| Remission status at HCT | ||||
| CR | 1.00 (reference) | — | ||
| Not in CR | 0.73 (0.39-1.35) | 0.32 | ||
| Conditioning | ||||
| BEAM | 1.00 (reference) | — | ||
| CBV | 0.59 (0.27-1.33) | 0.20 | 1.22 (0.54-2.79) | 0.63 |
| Other | 2.39 (0.99-5.79) | 0.053 | 3.04 (1.11-8.32) | 0.030 |
| PBSC mobilization regimen | ||||
| G-CSF only | 1.00 (reference) | — | ||
| G-CSF + cyclophosphamide | 0.80 (0.36-1.77) | 0.58 | ||
| G-CSF + plerixafor | 1.60 (0.72-3.58) | 0.25 | ||
| G-CSF + cyclophosphamide + plerixafor | 1.65 (0.77-3.55) | 0.20 | ||
| CD34 count | ||||
| >3 × 106 cells/kg | 1.00 (reference) | — | ||
| ≤3 × 106 cells/kg | 0.79 (0.35-1.78) | 0.57 | ||
| Pre-HCT CAD | 1.95 (0.68-5.56) | 0.21 | ||
| Hypertension | 2.66 (1.51-4.70) | 0.001 | 1.99 (1.10-3.62) | 0.023 |
| Diabetes | 1.66 (0.83-3.31) | 0.15 | ||
| Dyslipidemia | 1.18 (0.62-2.21) | 0.62 | ||
| Pre-HCT malignancy | 1.32 (0.62-2.84) | 0.48 | ||
CAD = coronary artery disease; HL = Hodgkin lymphoma; NHL = non-Hodgkin lymphoma; sHR = subdistribution HR; other abbreviations as in Table 1.
Test of heterogeneity P = 0.17.
Role of modifiable cardiovascular risk factors
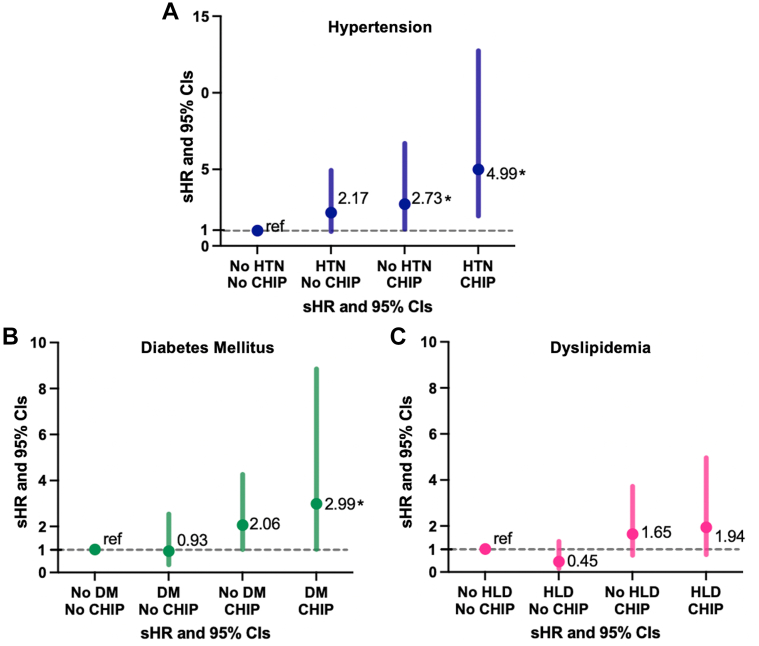
Hypertension was the most prevalent cardiovascular risk factor at HCT (32.1%), followed by dyslipidemia (24.5%) and diabetes (14.2%) (Table 1). There was a statistically significant, incremental increase in the cumulative incidence of HF across categories: no CHIP and no hypertension (3.2%), no CHIP and hypertension (8.5%), CHIP and no hypertension (11.2%), CHIP and hypertension (17.6%) (P < 0.001) (Supplemental Table 7). A similar trend was observed with diabetes, with the highest incidence (20.1%) among those with CHIP and diabetes (Supplemental Table 7). Of note, there was no statistically significant interaction between individual cardiovascular risk factors and CHIP. In the multivariable model adjusting for age, sex, HCT-specific comorbidity index, diagnosis, conditioning regimen, and respective cardiovascular risk factors, the highest magnitude of risk for HF was observed among patients with hypertension and CHIP (sHR: 4.99; 95% CI: 1.96-12.74) (Figure 3).
Figure 3.
Modifiable Cardiovascular Risk Factors, CHIP, and the Risk for Heart Failure
Subdistribution HRs (sHRs) and 95% CIs for heart failure outcomes according to the prespecified categories on the basis of clonal hematopoiesis of indeterminate potential (CHIP) and cardiovascular risk factor status (A, hypertension [HTN]; B, diabetes mellitus [DM]; C, dyslipidemia [HLD]), adjusted for age, sex, HCT-specific comorbidity index, diagnosis, conditioning regimen, and respective cardiovascular risk factors. ref = reference.
CHIP and survival after HCT
The 5-year overall survival rate for the cohort was 76.7%. Patients with CHIP had significantly worse 5-year overall survival (63.4% vs 80.3%; P < 0.001) compared with those without CHIP (Figure 4A). Patients with CHIP had a significantly greater incidence of NRM, with a comparable incidence of relapse-related mortality (Figures 4B and 4C). Among the 78 patients who died of nonrelapse causes, the proportions of cardiopulmonary and hematologic malignancy–related mortality were higher among patients with CHIP compared with those without CHIP (Supplemental Table 8). In the multivariable model, CHIP was significantly and independently associated with risk for all-cause mortality (HR: 1.41; 95% CI: 1.02-1.95), an association attributed to the much higher risk for NRM (sHR: 5.37; 95% CI: 2.34-12.35) compared with relapse-related mortality (sHR: 0.98; 95% CI: 0.68-1.41) (Supplemental Table 9).
Figure 4.
Survival Outcomes
(A) Cumulative incidence of all-cause mortality following HCT. (B, C) Cumulative incidence of cause-specific mortality following HCT according to the relapse status: (B) relapse-related mortality and (C) nonrelapse mortality. Abbreviations as in Figure 1.
Discussion
In this well-characterized and demographically diverse cohort of patients with lymphoma undergoing autologous HCT, CHIP was highly prevalent prior to HCT, occurring at a much higher rate than would be expected for the general population, and was significantly and independently associated with HF after HCT (Central Illustration). The incidence rate of HF increased by overall CHIP mutational burden, and the magnitude of risk was especially high among patients with both CHIP and hypertension. Patients harboring CHIP mutations had significantly worse survival compared with those without CHIP, driven by the markedly increased risk for NRM. The findings from this study highlight the potential role of pre-HCT CHIP as a novel biomarker and provide biologic insight that would allow refinement of HF risk characterization as well as considerations of early interventions to mitigate this risk.
Central Illustration.
CHIP and Risk of Heart Failure and Nonrelapse Mortality After Hematopoietic Cell Transplantation
DNA was extracted from cryopreserved mobilized peripheral blood stem cells of 861 patients who underwent autologous hematopoietic cell transplantation (HCT) for lymphoma. Targeted gene sequencing of isolated DNA revealed a rate of pre-HCT clonal hematopoiesis of indeterminate potential (CHIP) of 21.7%. The 5-year incidence of heart failure after HCT was significantly higher in patients with CHIP compared with those without CHIP (13.8% vs 4.7%; P < 0.001; subdistribution HR [sHR]: 2.48; 95% CI: 1.32-4.68). Patients with CHIP also had significantly worse overall survival after HCT, due primarily to the higher risk for nonrelapse mortality (sHR: 5.37; 95% CI: 2.34-12.35). ∗Created using BioRender.com.
Accumulating data in nononcology populations support our associations between CHIP and HF, including the incremental impact of CHIP mutational burden.15,16,27,28 In a meta-analysis involving 56,597 subjects without HF at baseline, patients with CHIP had a 25% increased risk for incident HF, independent of traditional risk factors.29 Notably, CHIP was not significantly associated with reduced EF. A separate study involving 8,090 subjects from 2 prospective nononcology cohort studies also found significant association only between TET2 CHIP and HF with preserved left ventricular EF but not reduced EF.30 Although the majority of patients with HF included in the present study had reduced EFs, there were a sizable proportion (40%) in which EF was preserved. Larger studies of patients with cancer with HF are needed to interrogate the association between CHIP and HF subtypes after treatment. In another study in patients with HF, CHIP was associated with significantly increased risk for all-cause and HF-specific death as well as HF-related hospitalization.27 The association between CHIP and HF did not differ by the underlying etiology of HF, namely, ischemic vs nonischemic, suggesting that CHIP may have a direct impact on the myocardium itself. In the present study, the association between CHIP and HF was independent of established risk factors in this population, such as older age, female sex, and high comorbidity burden at HCT. The magnitude of risk for HF in patients with CHIP was also much higher than that reported in nononcology populations. Surprisingly, unlike our previous study in patients undergoing autologous HCT from 1988 to 2002,9 we did not find an association between HF and pre-HCT anthracycline dose or chest RT. This may be due to the lower prevalence of chest RT (5.9% vs 12%) in this more contemporary era and a narrower range of anthracycline dose exposure in our more homogeneous cohort (lymphoma alone vs lymphoma, myeloma, or leukemia).9
It is also important to highlight that the mutational landscape of CHIP in our cohort differed from that reported in noncancer populations. Specifically, mutations in genes involved in DNA damage repair, such as PPM1D, TP53, and ATM, were more prevalent in our cohort compared with the general population.14 This observation is consistent with previous studies documenting an increased incidence of PPM1D and TP53 CHIP among patients with hematologic malignancies, likely driven by cytotoxic exposure that confers a survival advantage to these mutated cells.31 However, the strongest association with HF was seen with TET2 CHIP. This suggests gene-specific mechanisms that may contribute to HF risk and is an observation that is aligned with emerging preclinical and clinical data. In a study by Sano et al,32 competitive transplantation of Tet2-knockout bone marrow cells resulted in greater reduction of left ventricular function in a murine model of pressure overload achieved by transverse aortic constriction compared with wild-type transplantation. In a separate study examining the effect of adoptive transfer of Tet2-mutant bone marrow cells into nonirradiated mice mimicking the effects of CHIP,33 Tet2-mediated hematopoiesis resulted in significant cardiac dysfunction characterized by greater hypertrophy and fibrosis through interleukin-1β-mediated dysregulated inflammation. Similarly, accumulating preclinical data suggest how mutations in other commonly affected genes beside TET2 (eg, DNMT3A, PPM1D, TP53) contribute to HF, potentially elucidating the pathophysiological role of CHIP in HF through mechanisms such as inflammasome, fibrosis, and adverse cardiac remodeling, each driven by distinct gene-specific processes.34,35 As such, additional studies are warranted to first delineate how HF risk modulated by CHIP differs in the context of cancer-related exposures and whether patients harboring specific CHIP mutations may be at a heightened risk for HF.
Barring the development of CHIP-specific therapies, there are presently no effective treatments to mitigate the HF risk associated with CHIP. Thus, we turned our attention to the impact of modifiable cardiovascular risk factors such as hypertension, diabetes, and dyslipidemia. We found that patients with CHIP and hypertension had 5-fold risk for developing HF, and the 5-year cumulative incidence rate in patients with CHIP and diabetes exceeded 20%. Interestingly, we did not observe a significant trend with dyslipidemia, possibly because the etiology of HF in our cohort has historically been nonischemic in nature.26 The heightened HF risk among patients with hypertension or diabetes underscores the need to develop personalized risk management strategies, such as early screening and targeted treatment of these modifiable risk factors (eg, intensive blood pressure control, better glycemic control), a strategy that has been effectively used in the field of preventive cardiology for patients with germline risk for cardiovascular disease.36
Finally, our finding of worse survival among patients with CHIP compared with those without CHIP is aligned with existing data, which show worse survival in patients with CHIP after HCT that is attributable to the higher burden of NRM.29,37 Because of the relatively low proportion of NRM events (9% of the entire cohort), we were underpowered to conduct adjusted analyses for risk for cause-specific NRM. Nonetheless, the higher rates of cardiopulmonary and subsequent malignancy-related deaths among patients with CHIP speak to the greater contribution of these aging-related conditions to NRM, compared with those without CHIP.
Study limitations
First, despite limiting our CHIP panel to leukemogenic variants, some may represent circulating tumor DNA from lymphoma rather than CHIP. However, the majority of the identified CHIP mutations in our study are not hallmarks of lymphoma, and there was no statistically significant association between remission status at HCT and risk for CHIP. We also did not perform longitudinal sampling of blood to determine the extent to which clonal expansion of pre-HCT CHIP contributed to HF onset. Previous studies in nononcology populations have shown that CHIP with VAF ≥ 2% expands with time and is not lost, and this was partly the impetus for the chosen VAF threshold in the present study.38 Additionally, details regarding post-HCT blood pressure recordings and/or cardiovascular pharmacy data were not available. Therefore, we were unable to examine whether controlled vs uncontrolled blood pressure would differentially affect the risk for HF. Similarly, detailed information on smoking history as well as post-HCT incident coronary artery atherosclerosis, which could further influence the risk for HF, was not available for this study. Additionally, left ventricular EF data were not available for all patients, which limited our ability to classify HF subtypes, such as HF with reduced EF vs HF with preserved EF. Last, we recognize that our findings will need to be further examined in the context of patients with lymphoma with varying therapeutic exposures (eg, without HCT) and also validated in an independent cohort, setting the stage for broader integration of CHIP during and after lymphoma treatment.
Conclusions
We demonstrate a strong association between CHIP and the risk for HF after HCT in patients with lymphoma. This association was particularly pronounced in patients with concurrent CHIP and hypertension. Importantly, patients with CHIP had significantly worse survival outcomes, driven primarily by an elevated risk for NRM. These findings may guide more precise HF screening among patients with lymphoma being considered for HCT or after the completion of therapy, enabling personalized risk assessment and clinical management informed by biologically plausible biomarkers.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients with lymphoma undergoing autologous HCT, CHIP is highly prevalent and associated with significantly increased risk for HF as well as NRM.
TRANSLATIONAL OUTLOOK: Further studies are warranted to determine gene-specific mechanisms underlying CHIP-associated HF risk and to explore therapeutic strategies to mitigate this risk.
Funding Support and Author Disclosures
This work was supported by the V Foundation for Cancer Research (grant DT2019-006 to Dr Armenian). Drs Rhee and Armenian have received a research grant from Pfizer (unrelated to the present work). Dr Jamal has received research grants from Pfizer and Janssen (unrelated to the present work). Dr Natarajan has received grants from Allelica, Amgen, Apple, Boston Scientific, Genentech, and Novartis; is a consultant to Allelica, Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, HeartFlow, Novartis, Genentech, and GV; is a scientific advisory board member for Esperion Therapeutics, Preciseli, and TenSixteen Bio; and is a scientific cofounder of TenSixteen Bio; and his spouse is an employee of Vertex Pharmaceuticals (all unrelated to the present work). Dr Herrera has received consultancy and research funding from AstraZeneca, ADC Therapeutics, Genentech, Bristol Myers Squibb, Seagen, and Merck; has received consultancy fees from Tubulis, Takeda, and Karyopharm; and has received research funding from Kite, a Gilead Company, and Gilead Sciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Paaladinesh Thavendiranathan, MD, MSc, served as Guest Editor for this paper.
This study was presented in part at the 2023 annual meeting of the American Hematology Society as an oral abstract.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Copelan E.A. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Shah G.L., Moskowitz C.H. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood. 2018;131:1689–1697. doi: 10.1182/blood-2017-09-772673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza A., Fretham C., Lee S.J., et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–e182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe R.T., Advani R.H., Ai W.Z., et al. NCCN Guidelines® insights: Hodgkin lymphoma, version 2.2022. J Natl Compr Canc Netw. 2022;20:322–334. doi: 10.6004/jnccn.2022.0021. [DOI] [PubMed] [Google Scholar]
- 5.Georges G.E., Bar M., Onstad L., et al. Survivorship after autologous hematopoietic cell transplantation for lymphoma and multiple myeloma: late effects and quality of life. Biol Blood Marrow Transplant. 2020;26:407–412. doi: 10.1016/j.bbmt.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battiwalla M., Tichelli A., Majhail N.S. Long-term survivorship after hematopoietic cell transplantation: roadmap for research and care. Biol Blood Marrow Transplant. 2017;23:184–192. doi: 10.1016/j.bbmt.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmqvist A.S., Meng Q., Dai C., et al. Late morbidity and mortality after autologous blood or marrow transplantation for lymphoma in children, adolescents and young adults—a BMTSS report. Leukemia. 2024;38:601–609. doi: 10.1038/s41375-024-02144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S., Dai C., Landier W., et al. Trends in Late mortality and life expectancy after autologous blood or marrow transplantation over three decades: a BMTSS report. J Clin Oncol. 2022;40:1991–2003. doi: 10.1200/JCO.21.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian S.H., Sun C.L., Shannon T., et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasbinder A., Hoeger C.W., Catalan T., et al. Cardiovascular events after hematopoietic stem cell transplant: incidence and risk factors. JACC CardioOncol. 2023;5:821–832. doi: 10.1016/j.jaccao.2023.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker G.M., Thompson R.E., Hare J.M., et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 12.Steensma D.P., Bejar R., Jaiswal S., et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal S., Fontanillas P., Flannick J., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu B., Roberts M.B., Raffield L.M., et al. Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol. 2021;78:42–52. doi: 10.1016/j.jacc.2021.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochran J.D., Yura Y., Thel M.C., et al. Clonal hematopoiesis in clinical and experimental heart failure with preserved ejection fraction. Circulation. 2023;148:1165–1178. doi: 10.1161/CIRCULATIONAHA.123.064170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee J.W., Pillai R., He T., et al. Clonal hematopoiesis and cardiovascular disease in patients with multiple myeloma undergoing hematopoietic cell transplant. JAMA Cardiol. 2024;9:16–24. doi: 10.1001/jamacardio.2023.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouhieddine T.H., Sperling A.S., Redd R., et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat Commun. 2020;11:2996. doi: 10.1038/s41467-020-16805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayerhofer C., Sedrak M.S., Hopkins J.O., et al. Clonal hematopoiesis in older patients with breast cancer receiving chemotherapy. J Natl Cancer Inst. 2023;115:981–988. doi: 10.1093/jnci/djad065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson C.J., Lindsley R.C., Tchekmedyian V., et al. Clonal Hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armenian S.H., Yang D., Teh J.B., et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2:1756–1764. doi: 10.1182/bloodadvances.2018019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror M.L., Maris M.B., Storb R., et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson B.D., Fisher R.I., Barrington S.F., et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozkurt B., Hershberger R.E., Butler J., et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure) J Am Coll Cardiol. 2021;77:2053–2150. doi: 10.1016/j.jacc.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Li M.M., Datto M., Duncavage E.J., et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armenian S.H., Chow E.J. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120:469–479. doi: 10.1002/cncr.28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual-Figal D.A., Bayes-Genis A., Díez-Díez M., et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2021;77:1747–1759. doi: 10.1016/j.jacc.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Assmus B., Cremer S., Kirschbaum K., et al. Clonal haematopoiesis in chronic ischaemic heart failure: prognostic role of clone size for DNMT3A- and TET2-driver gene mutations. Eur Heart J. 2021;42:257–265. doi: 10.1093/eurheartj/ehaa845. [DOI] [PubMed] [Google Scholar]
- 29.Lackraj T., Ben Barouch S., Medeiros J.J.F., et al. Clinical significance of clonal hematopoiesis in the setting of autologous stem cell transplantation for lymphoma. Am J Hematol. 2022;97:1538–1547. doi: 10.1002/ajh.26726. [DOI] [PubMed] [Google Scholar]
- 30.Schuermans A., Honigberg M.C., Raffield L.M., et al. Clonal hematopoiesis and incident heart failure with preserved ejection fraction. JAMA Netw Open. 2024;7 doi: 10.1001/jamanetworkopen.2023.53244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florez M.A., Tran B.T., Wathan T.K., et al. Clonal hematopoiesis: mutation-specific adaptation to environmental change. Cell Stem Cell. 2022;29:882–904. doi: 10.1016/j.stem.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano S., Oshima K., Wang Y., et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Sano S., Yura Y., et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shumliakivska M., Luxán G., Hemmerling I., et al. DNMT3A clonal hematopoiesis-driver mutations induce cardiac fibrosis by paracrine activation of fibroblasts. Nat Commun. 2024;15:606. doi: 10.1038/s41467-023-43003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikking M.A., Stroeks S.L.V.M., Waring O.J., et al. Clonal hematopoiesis of indeterminate potential from a heart failure specialist’s point of view. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.123.030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navar A.M., Fine L.J., Ambrosius W.T., et al. Earlier treatment in adults with high lifetime risk of cardiovascular diseases: what prevention trials are feasible and could change clinical practice? Report of a National Heart, Lung, and Blood Institute (NHLBI) workshop. Am J Prev Cardiol. 2022;12 doi: 10.1016/j.ajpc.2022.100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Derkach A., Lewis N., et al. Clonal hematopoiesis in diffuse large B-cell lymphoma: clinical impact and genetic relatedness to lymphoma and therapy-related myeloid neoplasm. Haematologica. 2023;108:917–922. doi: 10.3324/haematol.2022.281724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin M.M., Zhou Y., Bick A.G., et al. Longitudinal profiling of clonal hematopoiesis provides insight into clonal dynamics. Immun Ageing. 2022;19:23. doi: 10.1186/s12979-022-00278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.