Abstract
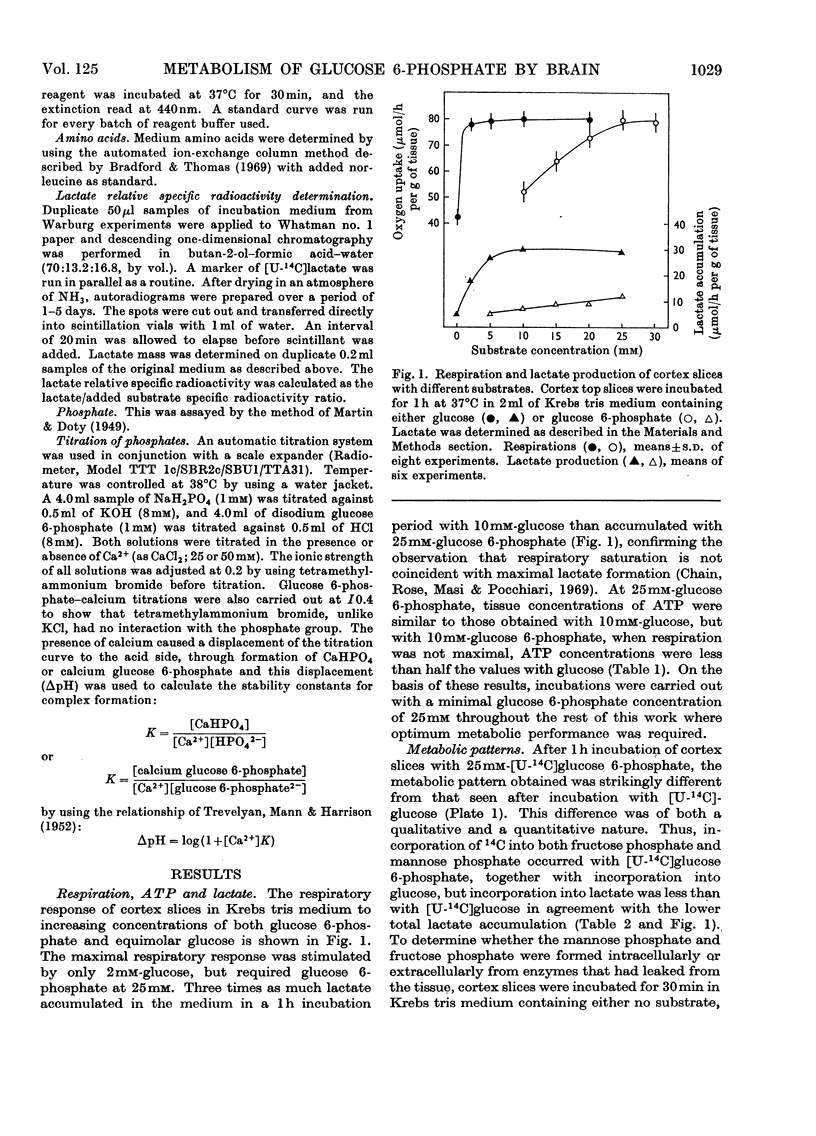
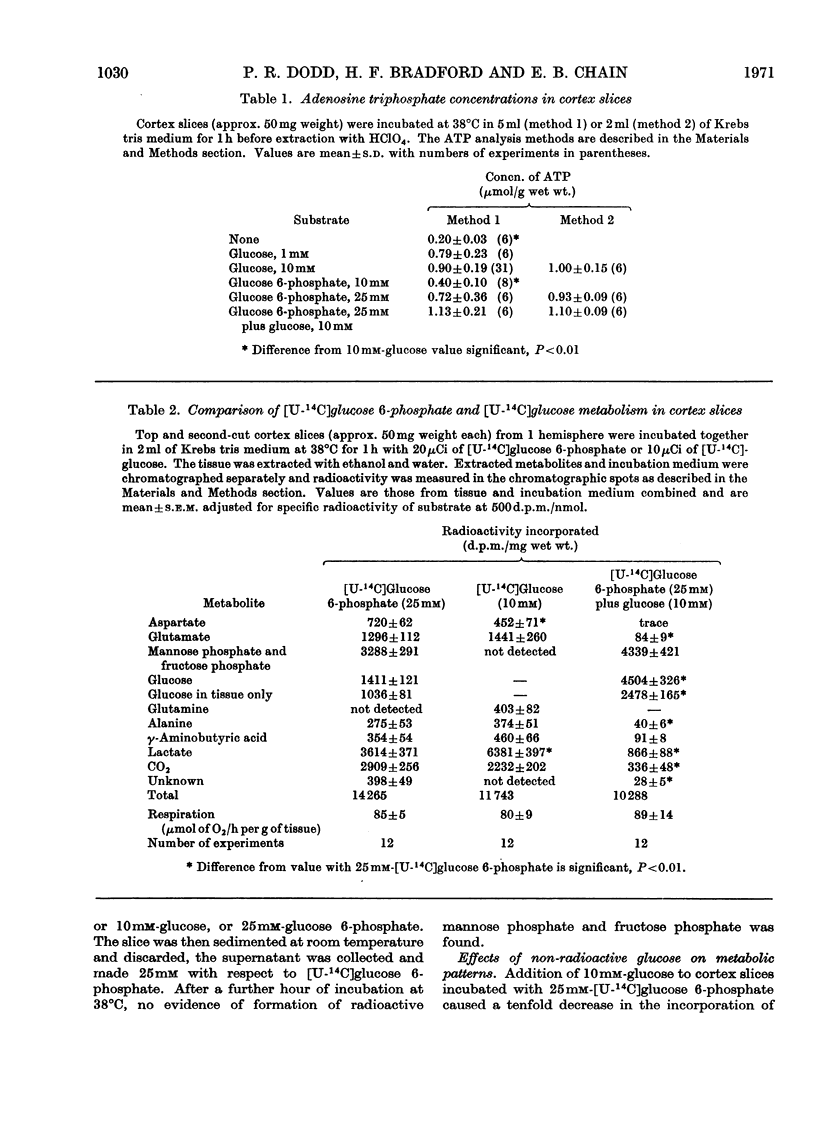
1. The metabolism of glucose 6-phosphate in rat cerebral-cortex slices in vitro was compared with that of glucose. It was found that a glucose 6-phosphate concentration of 25mm was required to achieve maximal oxygen uptake rates and ATP concentrations, whereas only 2mm-glucose was required. 2. When 25mm-[U-14C]glucose 6-phosphate was used as substrate, the pattern of labelling of metabolites was found to be quantitatively and qualitatively similar to the pattern found with 10mm-[U-14C]glucose, except that incorporation into [14C]lactate was decreased, and significant amounts of [14C]glucose and [14C]mannose phosphate and [14C]fructose phosphate were formed. 3. Unlabelled glucose (10mm) caused a tenfold decrease in the incorporation of 25mm-[U-14C]glucose 6-phosphate into all metabolites except [14C]glucose and [14C]mannose phosphate and [14C]fructose phosphate. In contrast, unlabelled glucose 6-phosphate (25mm) had no effect on the metabolism of 10mm-[U-14C]glucose other than to increase markedly the incorporation into, and amount of, [14C]lactate, the specific radioactivity of this compound remaining approximately the same. 4. The effect of glucose 6-phosphate in increasing lactate formation from glucose was found to occur also with a number of other phosphate esters and with inorganic phosphate. Further investigation indicated that the effect was probably due to binding of medium calcium by the phosphate moiety, thereby de-inhibiting glucose uptake. 5. Incubations carried out in a high-phosphate high-potassium medium gave a pattern of metabolism similar to that found when slices were subjected to depolarizing conditions. Tris-buffered medium gave similar results to bicarbonate-buffered saline, except that it allowed much less lactate formation from glucose. 6. Part of the glucose formed from glucose 6-phosphate was extracellular and was produced at a rate of 12μmol/h per g of tissue in Krebs tris medium when glycolysis was blocked. The amount formed was much less when 25mm-Pi or 26mm-HCO3− was present, the latter being in the absence of tris. 7. Glucose 6-phosphate also gave rise to an intracellular glucose pool, whereas no intracellular glucose was detectable when glucose was the substrate.
Full text
PDF


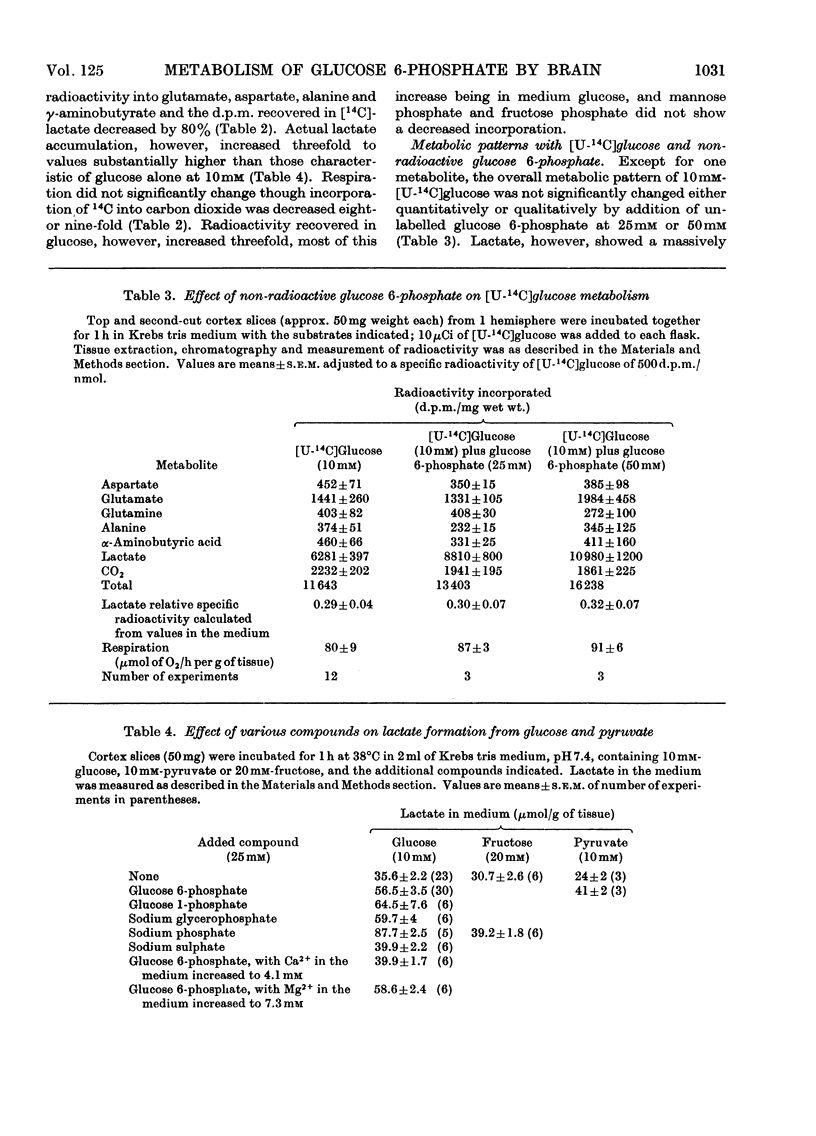
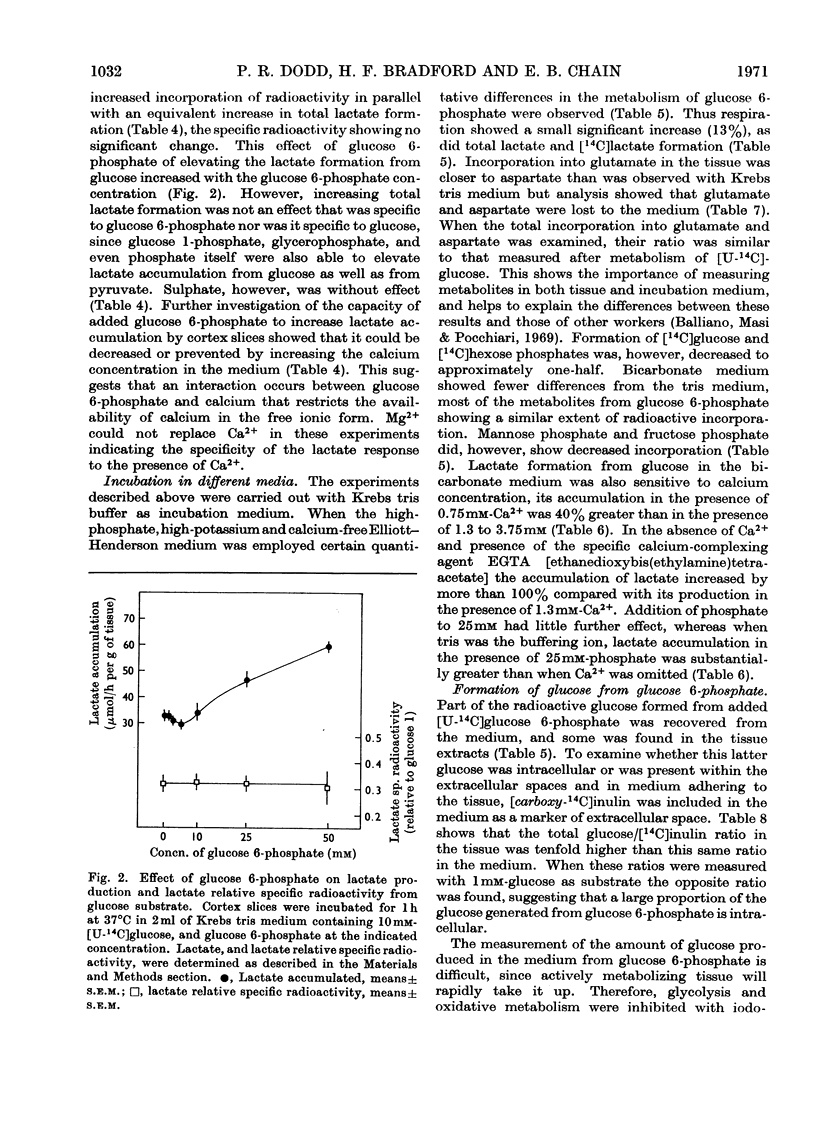
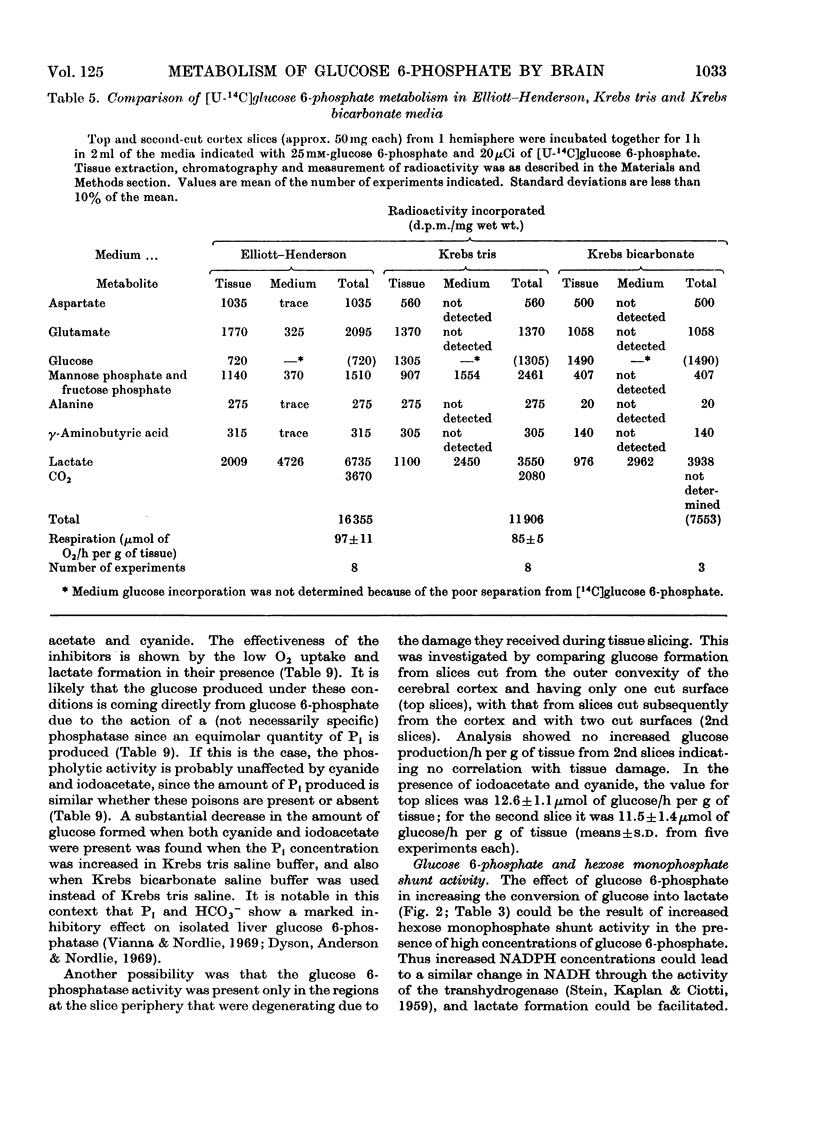
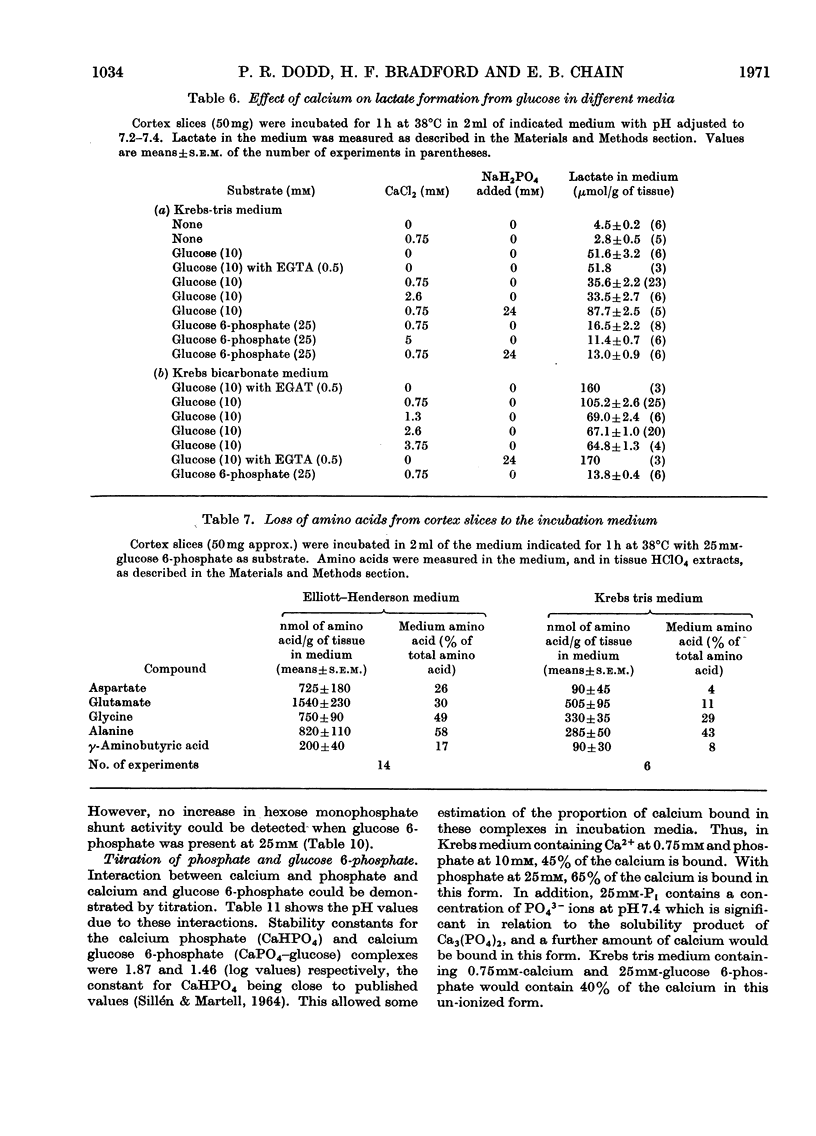
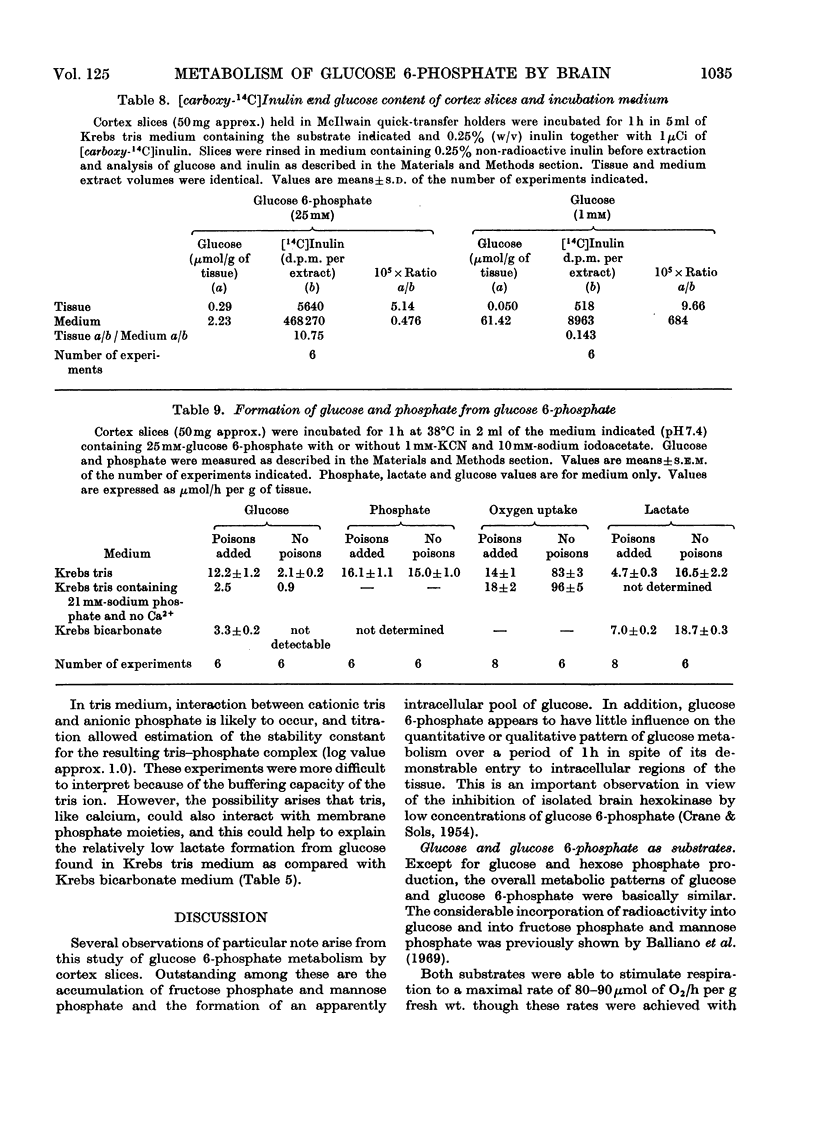



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnfred T., Hertz L. Effects of potassium and glutamate on brain cortex slices: uptake and release of glutamic and other amino acids. J Neurochem. 1971 Feb;18(2):259–265. doi: 10.1111/j.1471-4159.1971.tb00564.x. [DOI] [PubMed] [Google Scholar]
- BRUNS F. H., NOLTMANN E., WILLEMSEN A. Phosphomannose-isomerase. I. Uber die Aktivitätsmessung und die Sulfhydrylsowie die Metallabhängigkeit der Enzymwirkung in einigen tierischen Geweben. Biochem Z. 1958;330(5):411–420. [PubMed] [Google Scholar]
- Balliano M., Masi I., Pocchiari F. Metabolism of glucose-6-phosphate in slices of rat brain cortex. Brain Res. 1969 Mar;13(1):181–183. doi: 10.1016/0006-8993(69)90153-x. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Chain E. B., Cory H. T., Rose S. P. Glucose and amino acid metabolism in some invertebrate nervous systems. J Neurochem. 1969 Jun;16(3):969–978. doi: 10.1111/j.1471-4159.1969.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Bradford H. F., Thomas A. J. Metabolism of glucose and glutamate by synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 Nov;16(11):1495–1504. doi: 10.1111/j.1471-4159.1969.tb09904.x. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Chain E. B., Rose S. P., Masi I., Pocchiari F. Metabolism of hexoses in rat cerebral cortex slices. J Neurochem. 1969 Jan;16(1):93–100. doi: 10.1111/j.1471-4159.1969.tb10346.x. [DOI] [PubMed] [Google Scholar]
- Dyson J. E., Anderson W. B., Nordlie R. C. Inhibitory effect of physiological bicarbonate ion levels on the activities of glucose 6-phosphate phosphohydrolase. J Biol Chem. 1969 Feb 25;244(4):560–566. [PubMed] [Google Scholar]
- GIBSON I. M., MCILWAIN H. CONTINUOUS RECORDINGS OF CHANGES IN MEMBRANE POTENTIAL IN MAMMALIAN CEREBRAL TISSUES IN VITRO; RECOVERY AFTER DEPOLARIZATION BY ADDED SUBSTANCES. J Physiol. 1965 Jan;176:261–283. doi: 10.1113/jphysiol.1965.sp007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. K. The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J. 1960 Dec;77:610–618. doi: 10.1042/bj0770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiyama Y., Balázs R., Hammond B. J., Julian T., Richter D. The metabolism of gamma-aminobutyrate and glucose in potassium ion-stimulated brain tissue in vitro. Biochem J. 1970 Feb;116(3):469–481. doi: 10.1042/bj1160469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN A. M., KAPLAN N. O., CIOTTI M. M. Pyridine nucleotide transhydrogenase. VII. Determination of the reactions with coenzyme analogues in mammalian tissues. J Biol Chem. 1959 Apr;234(4):979–986. [PubMed] [Google Scholar]
- Sacks W., Sacks S. Conversion of glucose phosphate-14C to glucose-14C in passage through human brain in vivo. J Appl Physiol. 1968 Jun;24(6):817–827. doi: 10.1152/jappl.1968.24.6.817. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., MANN P. F. E., HARRISON J. S. The phosphorylase reaction. II. The influence of magnesium ions on equilibrium. Arch Biochem Biophys. 1952 Aug;39(2):440–449. doi: 10.1016/0003-9861(52)90352-4. [DOI] [PubMed] [Google Scholar]
- Vianna A. L., Nordlie R. C. The inhibition by physiological orthophosphate concentrations of hydrolytic and synthetic activities of liver glucose 6-phosphatase. J Biol Chem. 1969 Aug 10;244(15):4027–4032. [PubMed] [Google Scholar]