Abstract
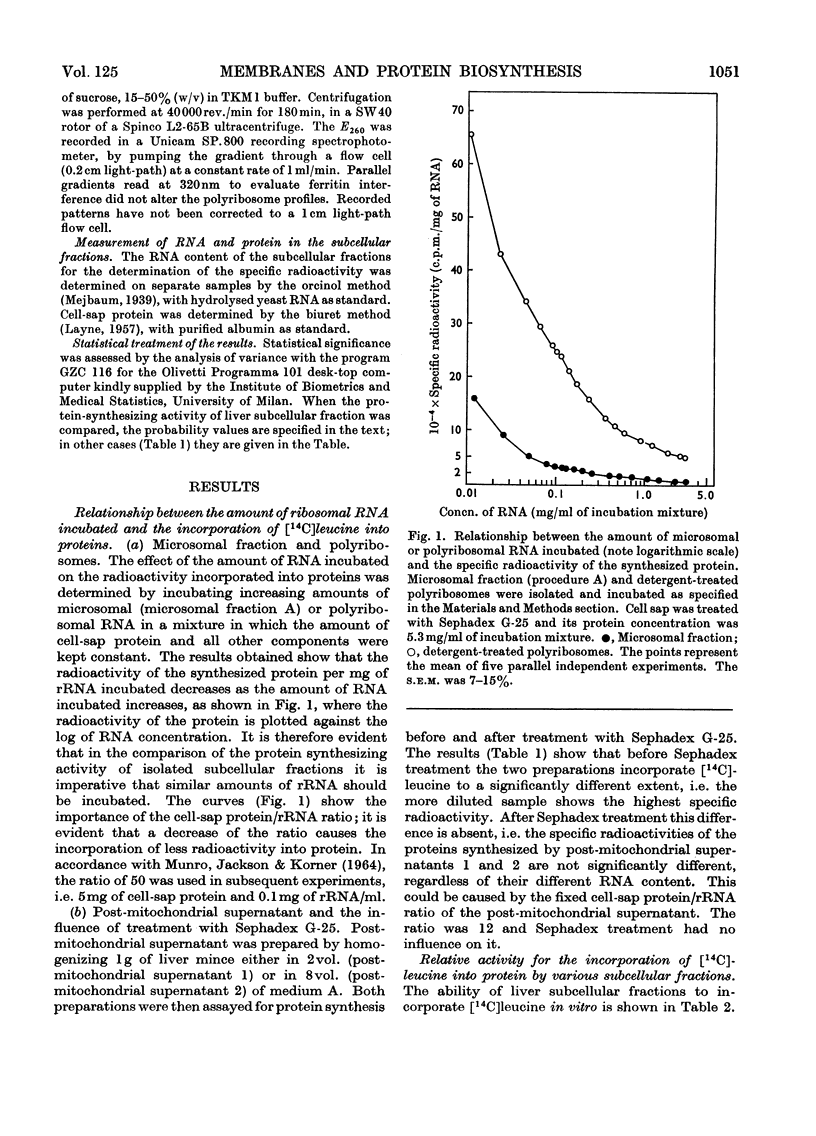
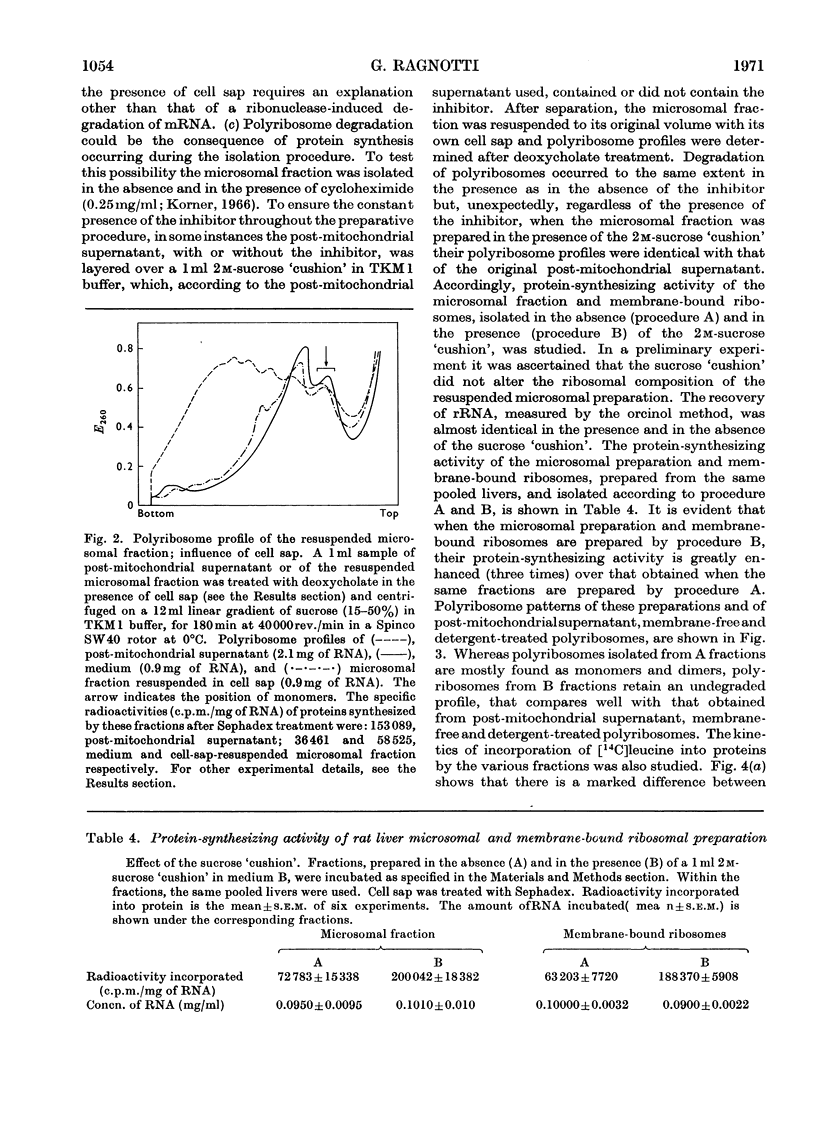
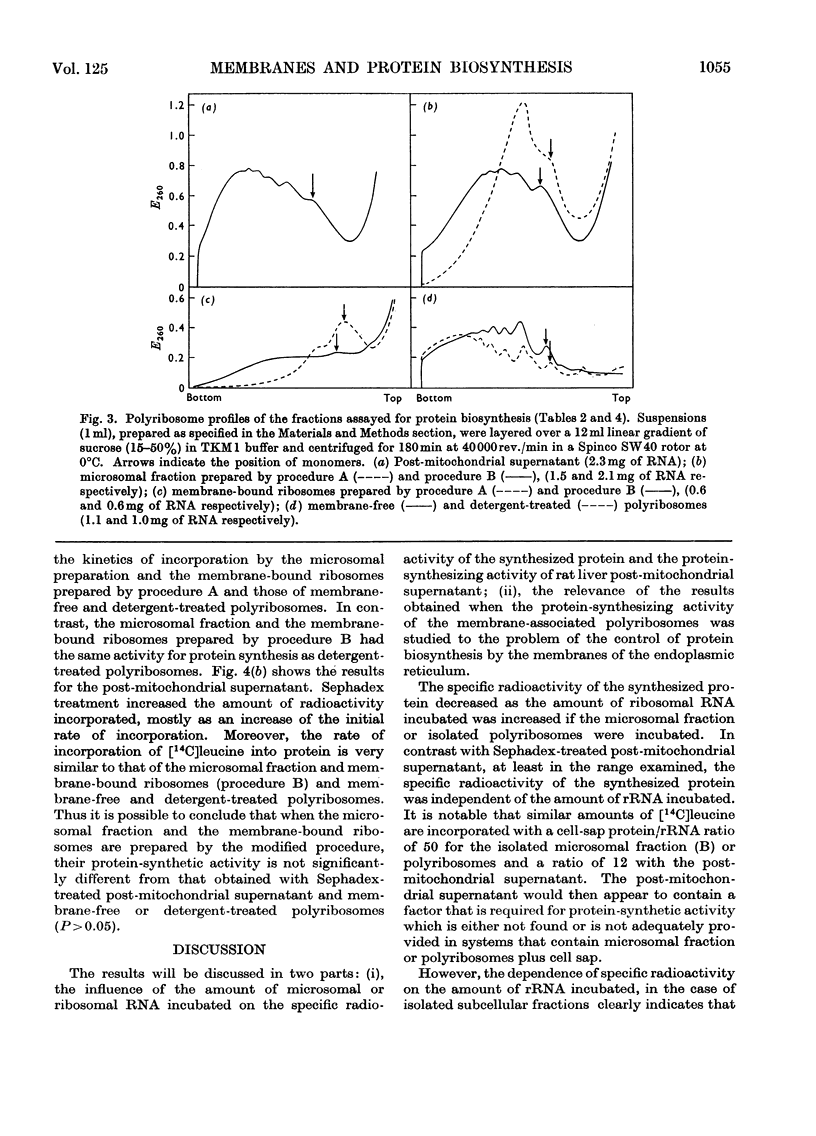
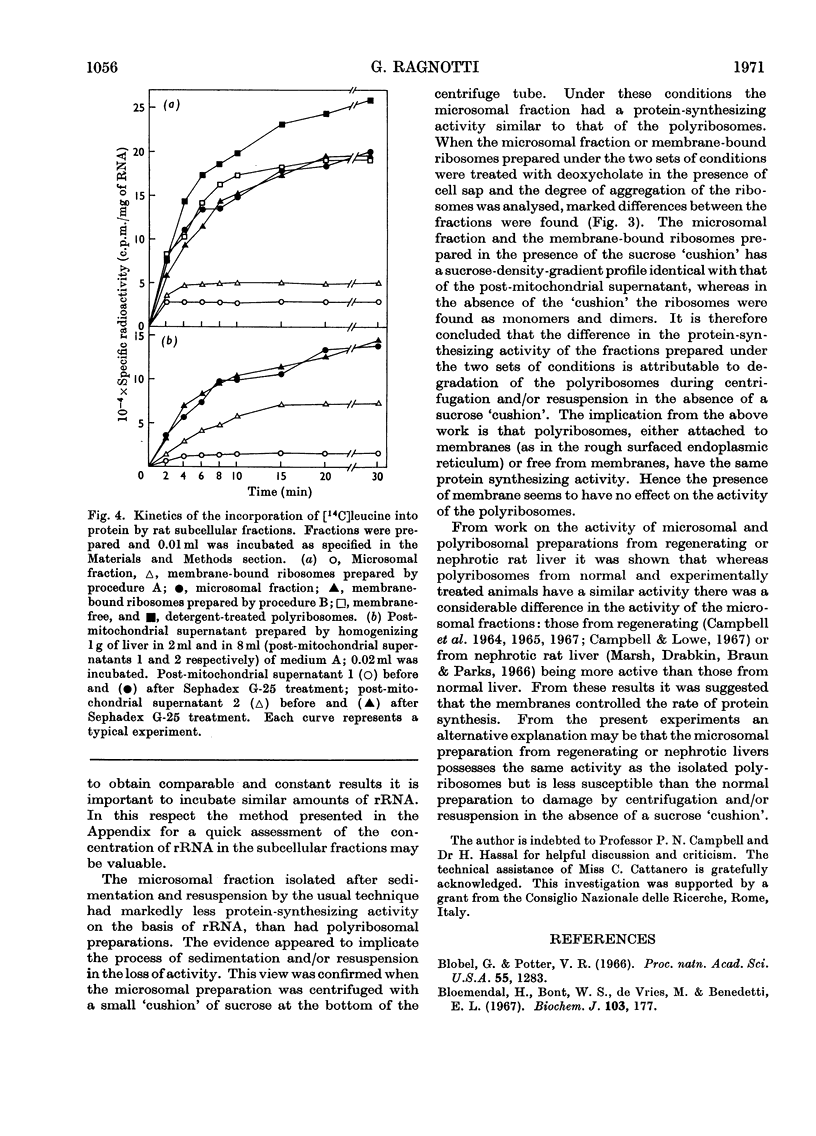
1. Various subcellular fractions containing ribosomes were isolated from rat liver. 2. In the presence of [14C]leucine and Sephadex-treated cell sap the radioactivity incorporated into the synthesized protein resulting from the incubation of microsomal preparations or deoxycholate-treated polyribosomes was dependent on the amount of rRNA incubated. In contrast, when Sephadex-treated post-mitochondrial supernatant was incubated, the radioactivity incorporated into the synthesized protein was independent of the amount of rRNA incubated. 3. Microsomal preparations and membrane-bound ribosomes, prepared by the standard procedure, incorporated less [14C]leucine into protein, per mg of rRNA incubated, than free or deoxycholate-treated polyribosomes; accordingly, polyribosomes associated with the former fractions were found mainly as monomers. 4. If microsomal fractions or membrane-bound ribosomes were prepared by a simple modification of the standard procedure, i.e. by centrifugation on to a `cushion' of 2m-sucrose, their protein-synthesizing activity was of the same order as that of the original post-mitochondrial supernatant, and membrane-free and deoxycholate-treated polyribosomes; in this case polyribosome profiles showed that very little degradation had occurred and compared well with those obtained for post-mitochondrial supernatant and isolated polyribosomes. 5. A method is described (Appendix) that provides a rapid and reliable assessment of the concentration of rRNA in subcellular fractions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Relation of ribonuclease and ribonuclease inhibitor to the isolation of polysomes from rat liver. Proc Natl Acad Sci U S A. 1966 May;55(5):1283–1288. doi: 10.1073/pnas.55.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., de Vries M., Benedetti E. L. Isolation and properties of polyribosomes and fragments of the endoplasmic reticulum from rat liver. Biochem J. 1967 Apr;103(1):177–182. doi: 10.1042/bj1030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Cooper C., Hicks M. Studies on the role of the morphological constituents of the microsome fraction from rat liver in protein synthesis. Biochem J. 1964 Aug;92(2):225–234. doi: 10.1042/bj0920225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Lowe E., Serck-Hanssen G. Protein synthesis by microsomal particles from regenerating rat liver. Biochem J. 1967 Apr;103(1):280–288. doi: 10.1042/bj1030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Serck-Hanssen G., Lowe E. Studies on the protein-synthesizing activity of the ribosomes of rat liver. The activity of free polysomes. Biochem J. 1965 Nov;97(2):422–431. doi: 10.1042/bj0970422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner A. Effect of cycloheximide on protein biosynthesis in rat liver. Biochem J. 1966 Dec;101(3):627–631. doi: 10.1042/bj1010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford G. R., Langford P., Schachter H. The inhibition of rat liver polyribosome breakdown in the presence of liver supernatant. J Biol Chem. 1966 Apr 25;241(8):1835–1839. [PubMed] [Google Scholar]
- Macdonald R. I., Korner A. Growth hormone stimulation of protein synthetic activity of membrane-bound ribosomes. FEBS Lett. 1971 Feb 12;13(1):62–64. doi: 10.1016/0014-5793(71)80665-8. [DOI] [PubMed] [Google Scholar]
- Marsh J. B., Drabkin D. L., Braun G. A., Parks J. S. Factors in the stimulation of protein synthesis by subcellular preparations from rat liver. J Biol Chem. 1966 Sep 25;241(18):4168–4174. [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnotti G., Cajone F., Bernelli-Zazzera A. Structural and functional changes in polysomes from ischemic livers. Exp Mol Pathol. 1970 Dec;13(3):295–306. doi: 10.1016/0014-4800(70)90092-4. [DOI] [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]


