Abstract
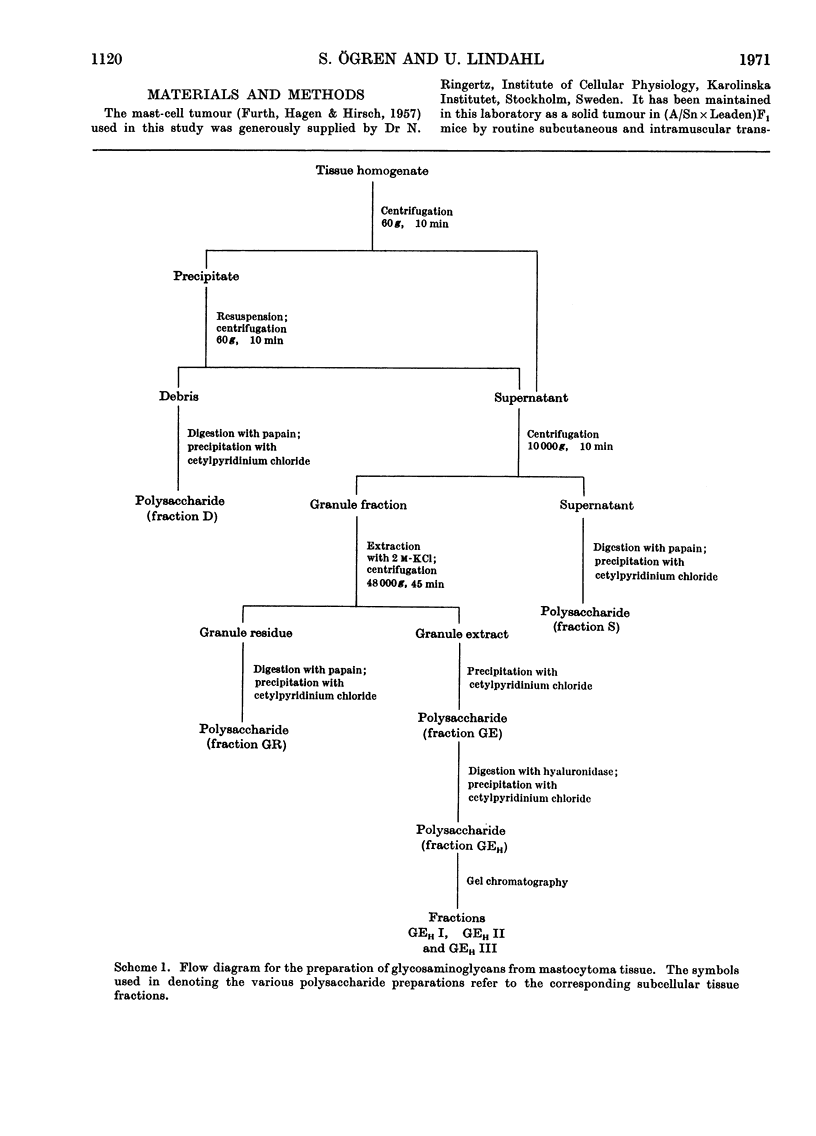
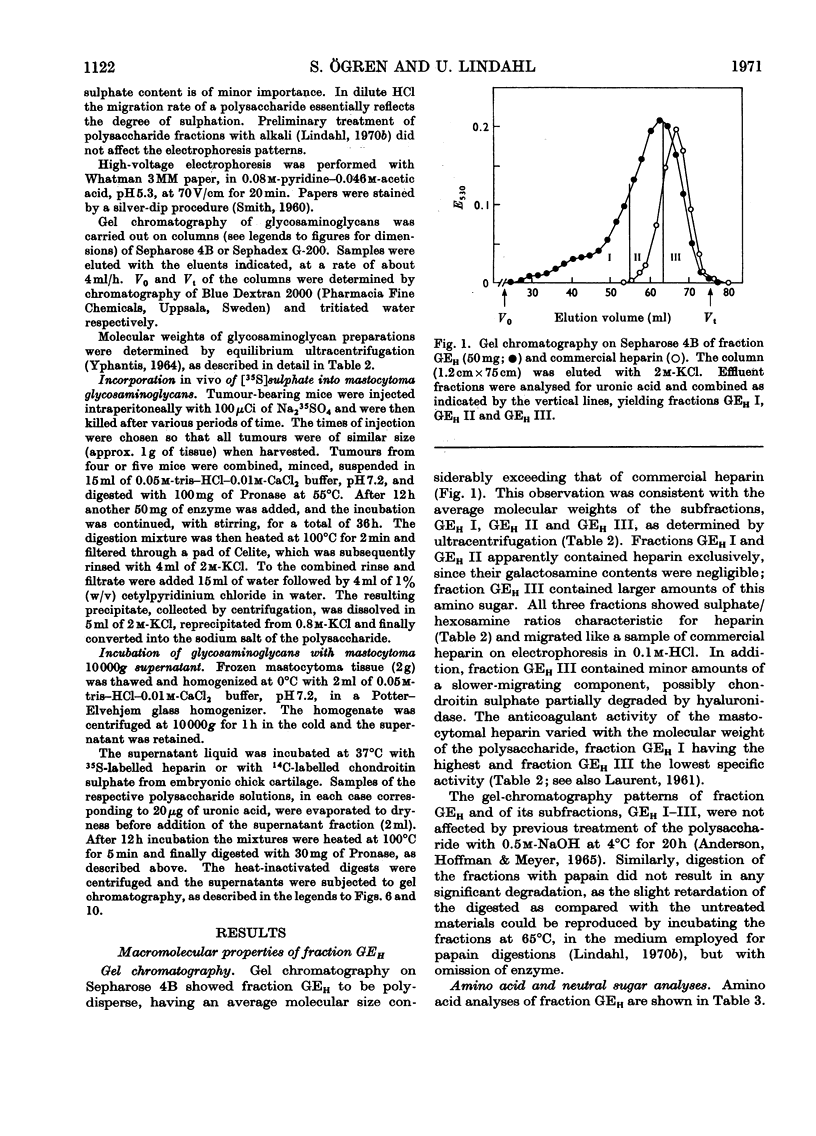
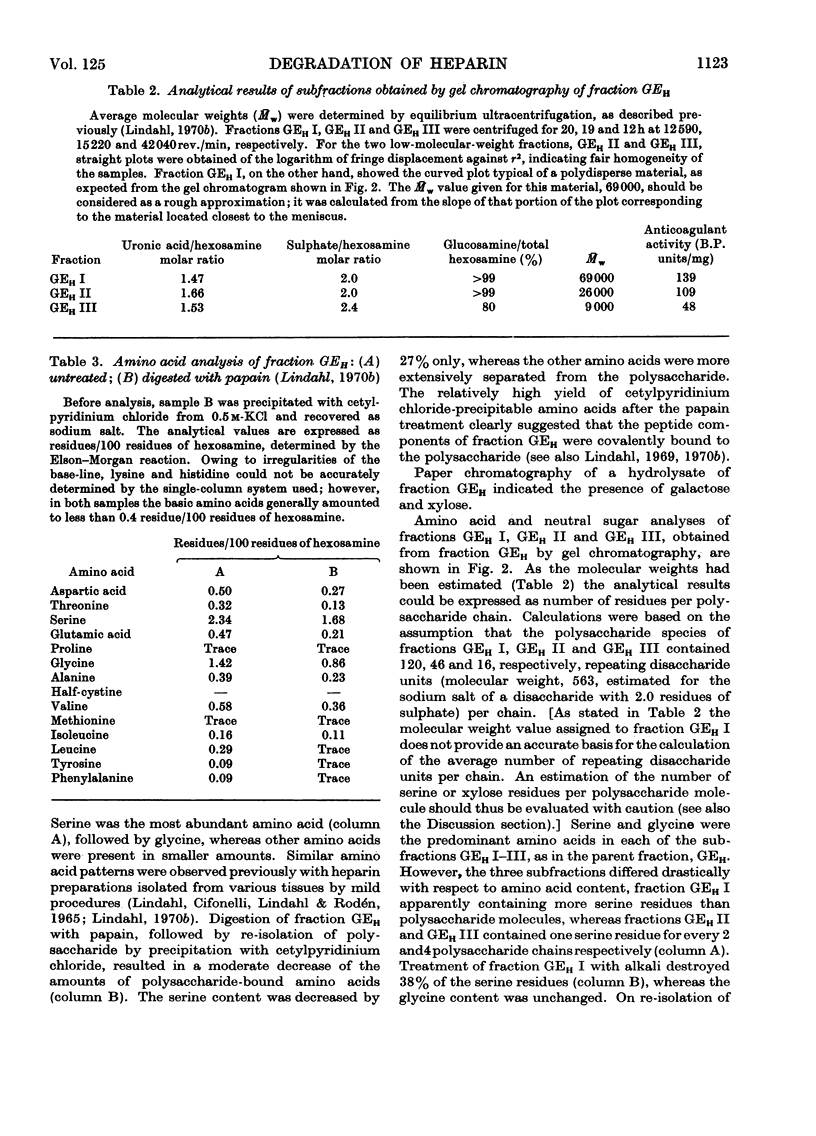
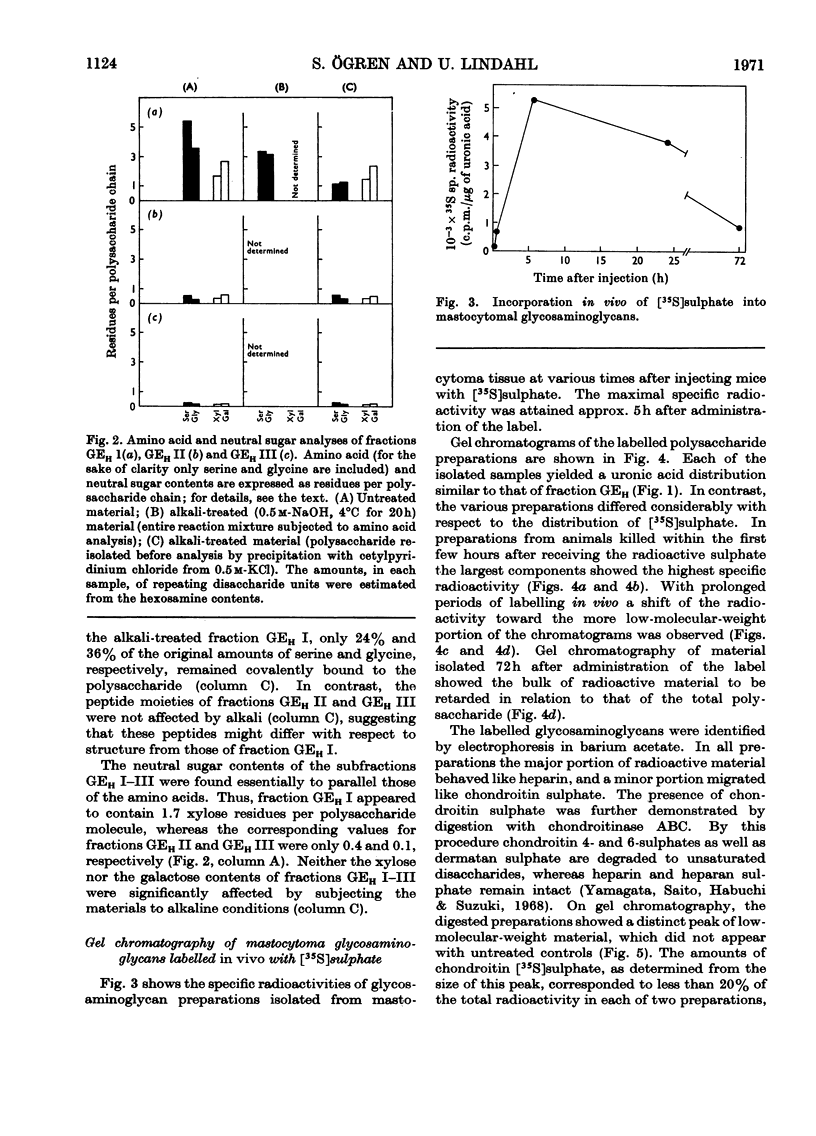
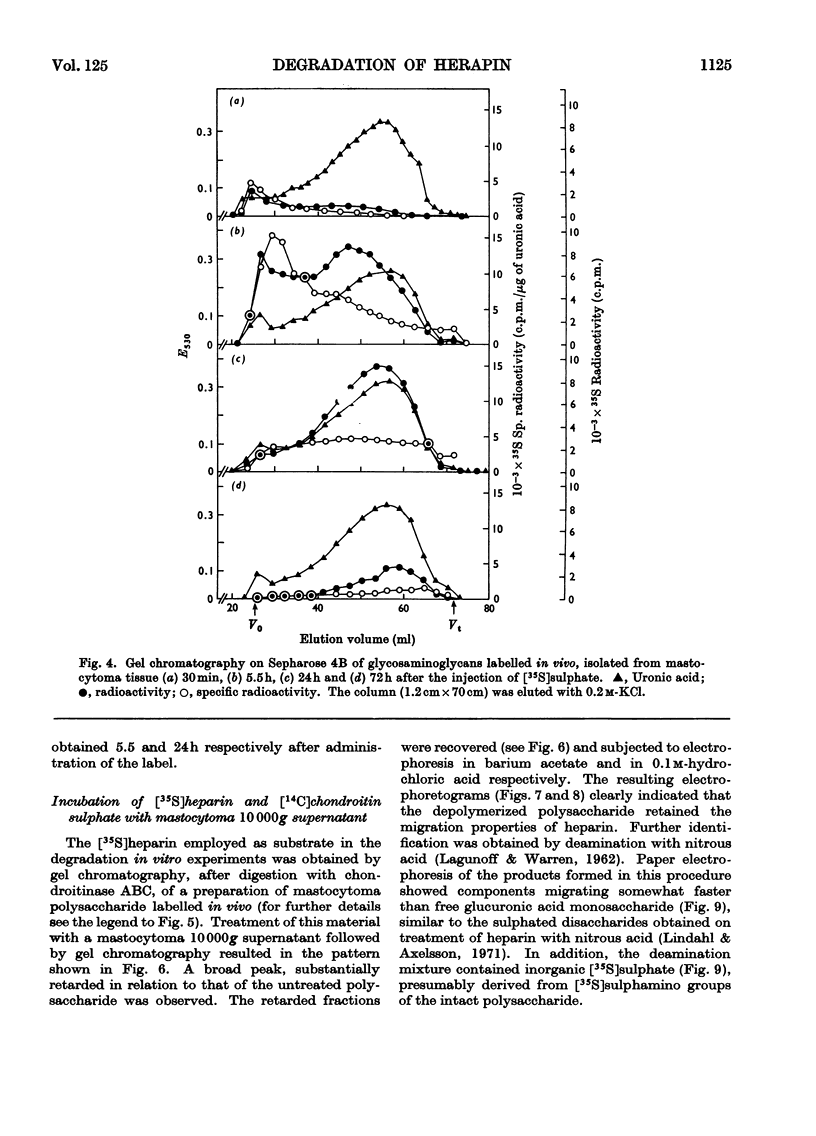
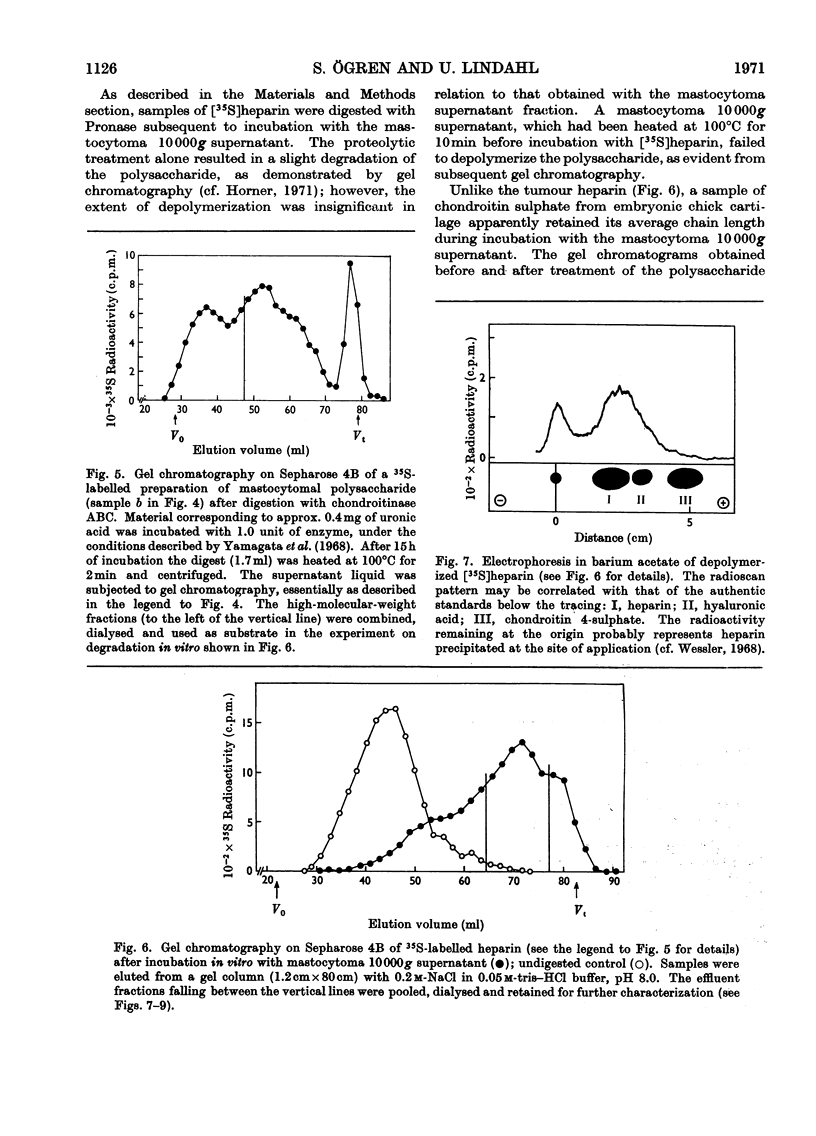
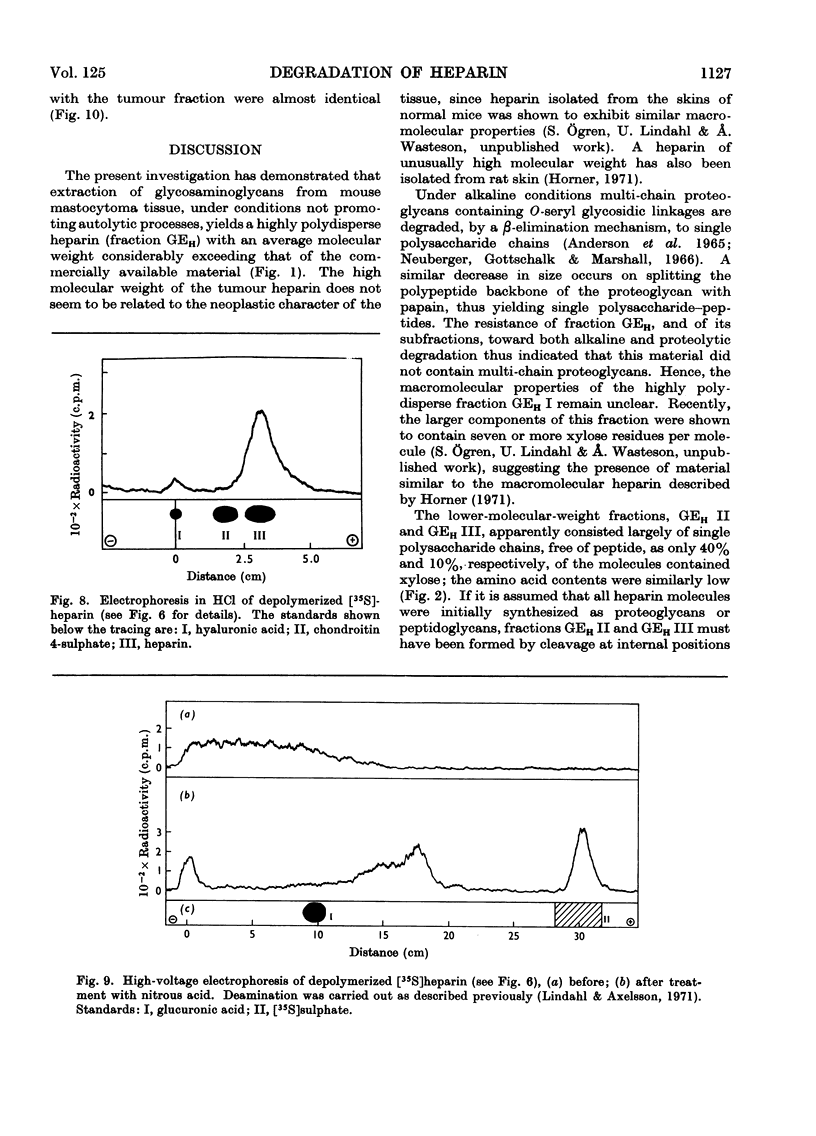
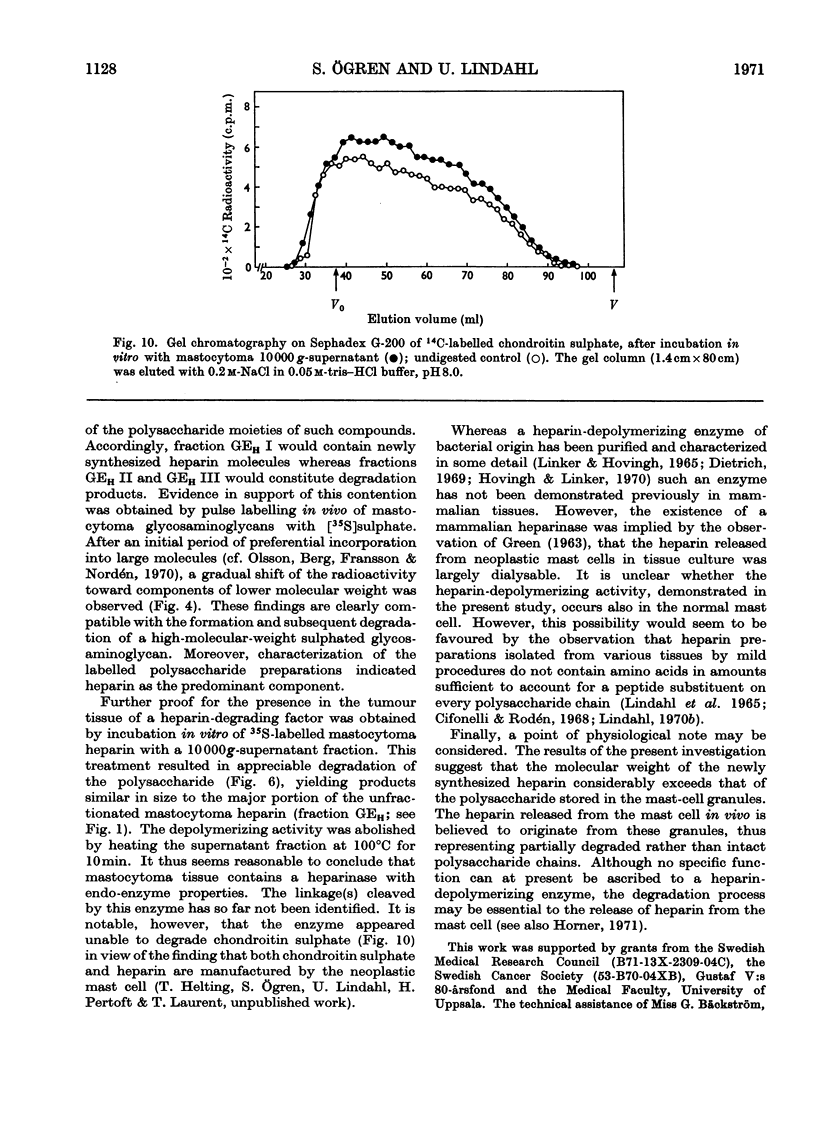
1. Heparin was prepared from mouse mastocytoma tissue by mild procedures, including extraction of mast-cell granules with 2m-potassium chloride, precipitation of the extracted polysaccharide with cetylpyridinium chloride from 0.8m-potassium chloride and finally digestion of the isolated material with testicular hyaluronidase. The resulting product (fraction GEH) represented approx. 40% of the total heparin content of the tissue. 2. Fraction GEH was fractionated by gel chromatography on Sepharose 4B into three subfractions, with average molecular weights (¯Mw) of approx. 60000–70000 (highly polydisperse material), 26000 and 9000 respectively. Treatment of each of the subfractions with alkali or with papain did not affect their behaviour on gel chromatography. Amino acid and neutral sugar analyses indicated that the two low-molecular-weight fractions consisted largely of single polysaccharide chains lacking the carbohydrate–protein linkage region. It was suggested that these heparin molecules had been degraded by an endopolysaccharidase. 3. Pulse labelling in vivo of mastocytoma heparin with [35S]sulphate showed initial labelling of large molecules followed by a progressive shift of radioactivity toward fractions of lower molecular weight. Further, heparin-depolymerizing activity was demonstrated by incubating 35S-labelled heparin in vitro with a mastocytoma 10000g-supernatant fraction. Appreciable degradation of the polysaccharide occurred, as demonstrated by gel chromatography. In contrast, no depolymerization was observed on subjecting 14C-labelled chondroitin sulphate to the same procedure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Davidson E. A. Catabolism of mucopolysaccharides by rat liver lysosomes in vivo. J Biol Chem. 1968 Sep 10;243(17):4494–4499. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P. Enzymic degradation of heparin. A glucosaminidase and a glycuronidase from Flavobacterium heparinum. Biochemistry. 1969 May;8(5):2089–2094. doi: 10.1021/bi00833a046. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURTH J., HAGEN P., HIRSCH E. I. Transplantable mastocytoma in the mouse containing histamine, heparin, 5-hydroxytryptamine. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):824–828. doi: 10.3181/00379727-95-23375. [DOI] [PubMed] [Google Scholar]
- GREEN J. P. Elimination and catabolism of 35S-heparin by neoplastic mast cells in culture. Biochem Pharmacol. 1963 Jun;12:588–590. doi: 10.1016/0006-2952(63)90137-0. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Macromolecular heparin from rat skin. Isolation, characterization, and depolymerization with ascorbate. J Biol Chem. 1971 Jan 10;246(1):231–239. [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- Jansson L., Lindahl U. Evidence for the existence of a multichain proteoglycan of heparan sulphate. Biochem J. 1970 May;117(4):699–702. doi: 10.1042/bj1170699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D. The isolation of heparin from mouse mast cell tumor. J Biol Chem. 1959 Jun;234(6):1325–1329. [PubMed] [Google Scholar]
- LAGUNOFF D., WARREN G. Determination of 2-deoxy-2-sulfoaminohexose content of mucopolysaccharides. Arch Biochem Biophys. 1962 Dec;99:396–400. doi: 10.1016/0003-9861(62)90285-0. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C. Studies on fractionated heparin. Arch Biochem Biophys. 1961 Feb;92:224–231. doi: 10.1016/0003-9861(61)90341-1. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970 Jan;116(1):27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Axelsson O. Identification of iduronic acid as the major sulfated uronic acid of heparin. J Biol Chem. 1971 Jan 10;246(1):74–82. [PubMed] [Google Scholar]
- Lindahl U. Comment on the use of cetylpyridinium chloride in the isolation of connective-tissue proteoglycan. Biochem J. 1969 Jul;113(3):569–570. doi: 10.1042/bj1130569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U. Further characterization of the heparin-protein linkage region. Biochim Biophys Acta. 1966 Dec 28;130(2):368–382. doi: 10.1016/0304-4165(66)90233-9. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The enzymatic degradation of heparin and heparitin sulfate. I. The fractionation of a crude heparinase from flavobacteria. J Biol Chem. 1965 Oct;240(10):3724–3728. [PubMed] [Google Scholar]
- Olsson I., Berg B., Fransson L. A., Nordén A. The identity of the metachromatic substance of basophilic leucocytes. Scand J Haematol. 1970;7(6):440–444. doi: 10.1111/j.1600-0609.1970.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Platt D., Stein U. Untersuchungen zum katabolen Mucopolysaccharid-Protein-Stoffwechsel in menschlichen Organen. Z Klin Chem Klin Biochem. 1969 Jul;7(4):374–378. [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


