Abstract
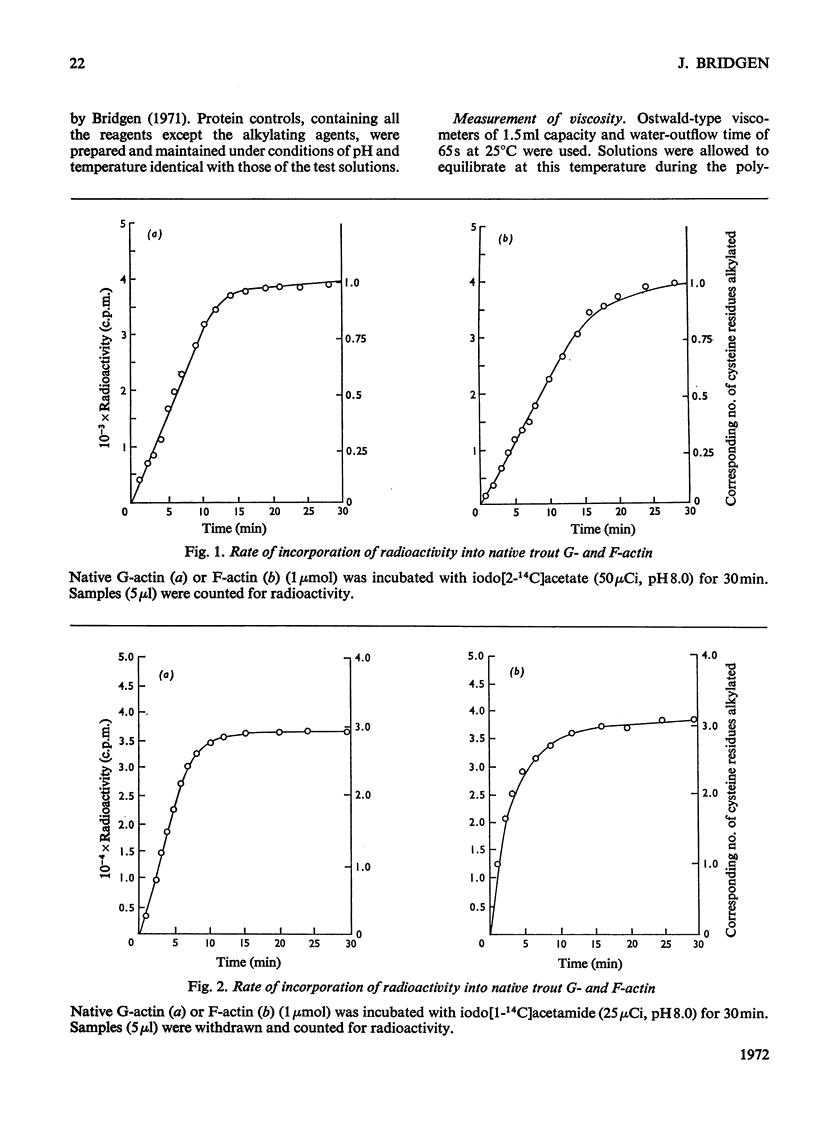
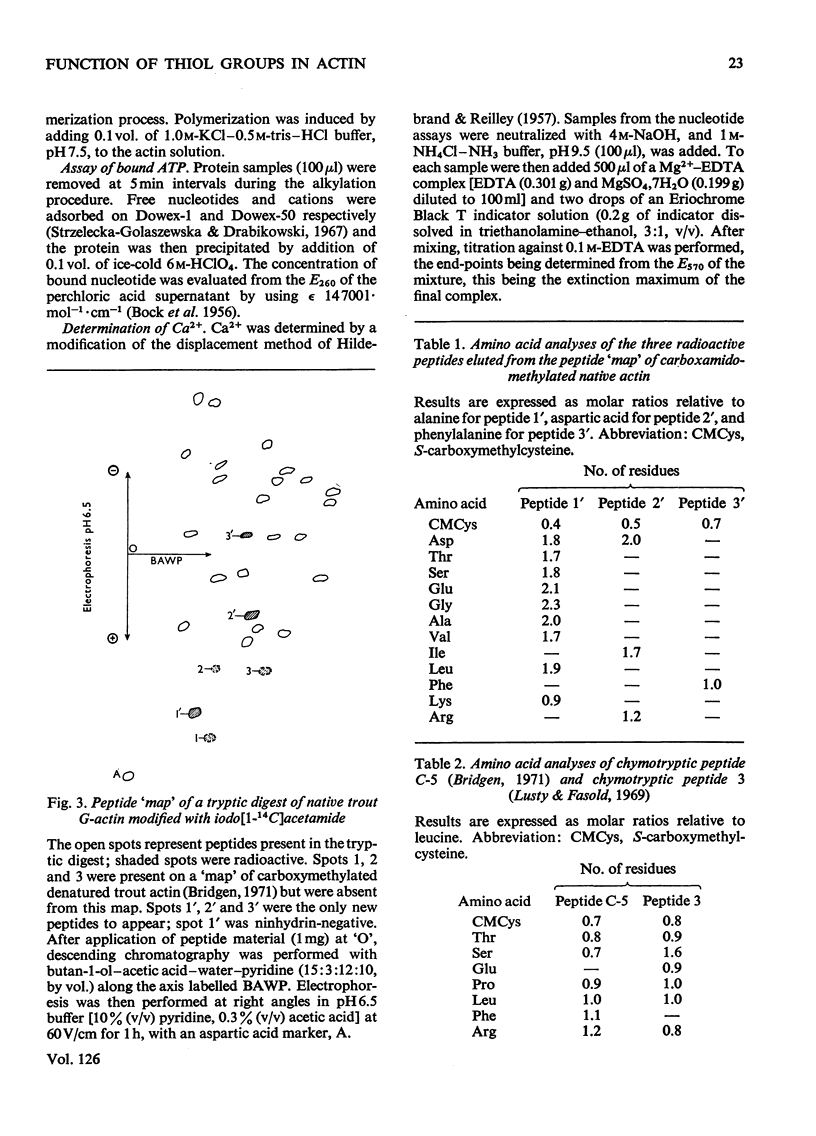
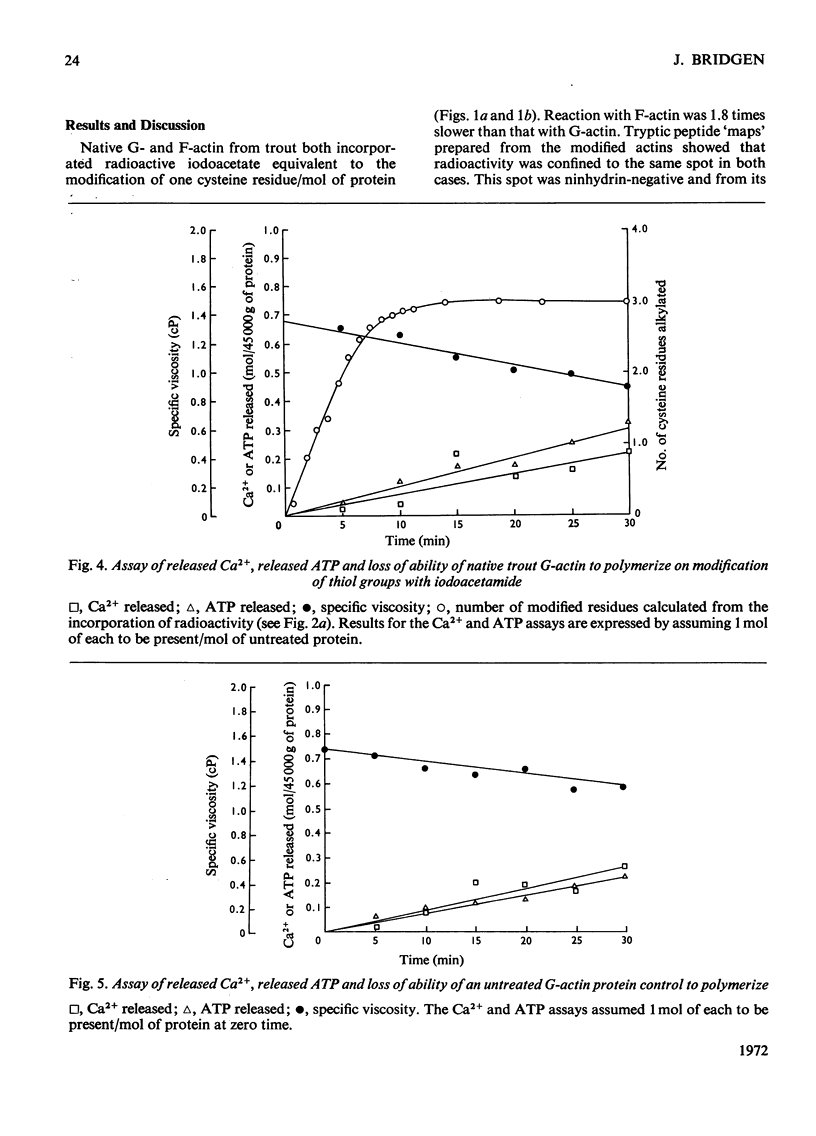
1. Considerable differences were found between the rates and degrees of modification of native trout actin with iodo[2-14C]acetate and iodo[1-14C]acetamide. 2. With iodoacetate, G- and F-actin were both labelled in the N-terminal peptide only. This modification had little effect on the ability of the actin to polymerize. 3. Iodoacetamide labelled three cysteine residues in both G- and F-actin. The modified cysteine residues were identified from the position of the corresponding tryptic peptides on peptide `maps'. 4. The modification had little effect on the ability of G-actin to polymerize, to bind ATP or to bind Ca2+, or on the ability of F-actin to depolymerize. 5. It is concluded that the three cysteine residues present on the `surface' of the native trout actin molecule have no direct role in the polymerization processes, the binding of ATP, or the binding of Ca2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAKURA S. The interaction between G-actin and ATP. Arch Biochem Biophys. 1961 Jan;92:140–149. doi: 10.1016/0003-9861(61)90228-4. [DOI] [PubMed] [Google Scholar]
- Adelstein R. S., Kuehl W. M. Structural studies on rabbit skeletal actin. I. Isolation and characterization of the peptides produced by cyanogen bromide cleavage. Biochemistry. 1970 Mar 17;9(6):1355–1364. doi: 10.1021/bi00808a009. [DOI] [PubMed] [Google Scholar]
- BARANY M. Studies on the actin-actin bonding. Biochim Biophys Acta. 1956 Mar;19(3):560–562. doi: 10.1016/0006-3002(56)90488-7. [DOI] [PubMed] [Google Scholar]
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- Bailin G., Bárány M. Studies on actin-actin and actin-myosin interaction. Biochim Biophys Acta. 1967 Jun 27;140(2):208–221. doi: 10.1016/0005-2795(67)90461-8. [DOI] [PubMed] [Google Scholar]
- Bridgen J. The amino acid sequence around four cysteine residues in trout actin. Biochem J. 1971 Jul;123(4):591–600. doi: 10.1042/bj1230591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M. Amino acid sequence studies on rabbit skeletal muscle actin. Cyanogen bromide cleavage of the protein and determination of the sequences of seven of the resulting peptides. Biochemistry. 1970 Mar 17;9(6):1365–1374. doi: 10.1021/bi00808a010. [DOI] [PubMed] [Google Scholar]
- Johnson P., Perry S. V. Chemical studies on the cysteine and terminal peptides in tryptic digests of actin. Biochem J. 1968 Nov;110(2):207–216. doi: 10.1042/bj1100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M., MOMMAERTS W. F. The sulfhydryl groups of actin. Biochim Biophys Acta. 1962 Nov 19;65:82–92. doi: 10.1016/0006-3002(62)90151-8. [DOI] [PubMed] [Google Scholar]
- KUSCHINSKY G., TURBA F. Uber die Rolle der SH-Gruppen bei Vorgängen am Aktomyosin, Myosin und Aktin. Biochim Biophys Acta. 1951 Jan;6(3):426–433. doi: 10.1016/0006-3002(50)90114-4. [DOI] [PubMed] [Google Scholar]
- Kuehl W. M., Gergely J. The kinetics of exchange of adenosine triphosphate and calcium with G-actin. J Biol Chem. 1969 Sep 10;244(17):4720–4729. [PubMed] [Google Scholar]
- Lusty C. J., Fasold H. Characterization of sulfhydryl groups of actin. Biochemistry. 1969 Jul;8(7):2933–2939. doi: 10.1021/bi00835a036. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., GOUVEA M. A. Studies on actin. V. Chemical modification of actin. J Biol Chem. 1961 May;236:1338–1344. [PubMed] [Google Scholar]
- MARUYAMA K., GERGELY J. Removal of the bound calcium of G-actin by ethylenediaminetetraacetate (EDTA). Biochem Biophys Res Commun. 1961 Nov 29;6:245–249. doi: 10.1016/0006-291x(61)90371-0. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. The action of thiol inhibitors on the interaction of F-actin and heavy meromyosin. Biochem J. 1964 Sep;92(3):603–608. doi: 10.1042/bj0920603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- STROHMAN R. C., SAMORODIN A. J. The requirements for adenosine triphosphate binding to globular actin. J Biol Chem. 1962 Feb;237:363–370. [PubMed] [Google Scholar]
- Seraydarian K., Briskey E., Mommaerts W. F. The modification of actomyosin by alpha-actinin. IV. The role of sulfhydryl groups. Biochim Biophys Acta. 1968 Oct 1;162(3):424–434. doi: 10.1016/0005-2728(68)90128-x. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Golaszewska H., Drabikowski W. Correlation between the binding of calcium and ATP by G-actin. Acta Biochim Pol. 1967;14(2):195–208. [PubMed] [Google Scholar]


