Abstract
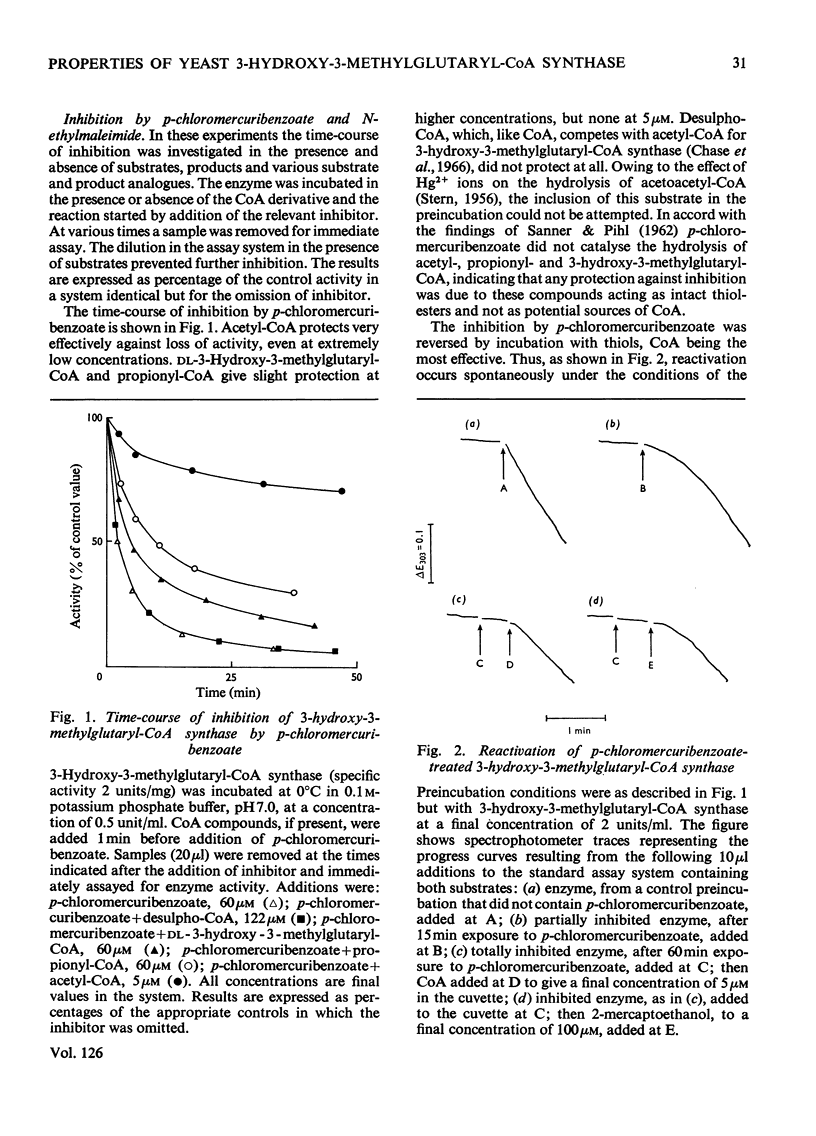
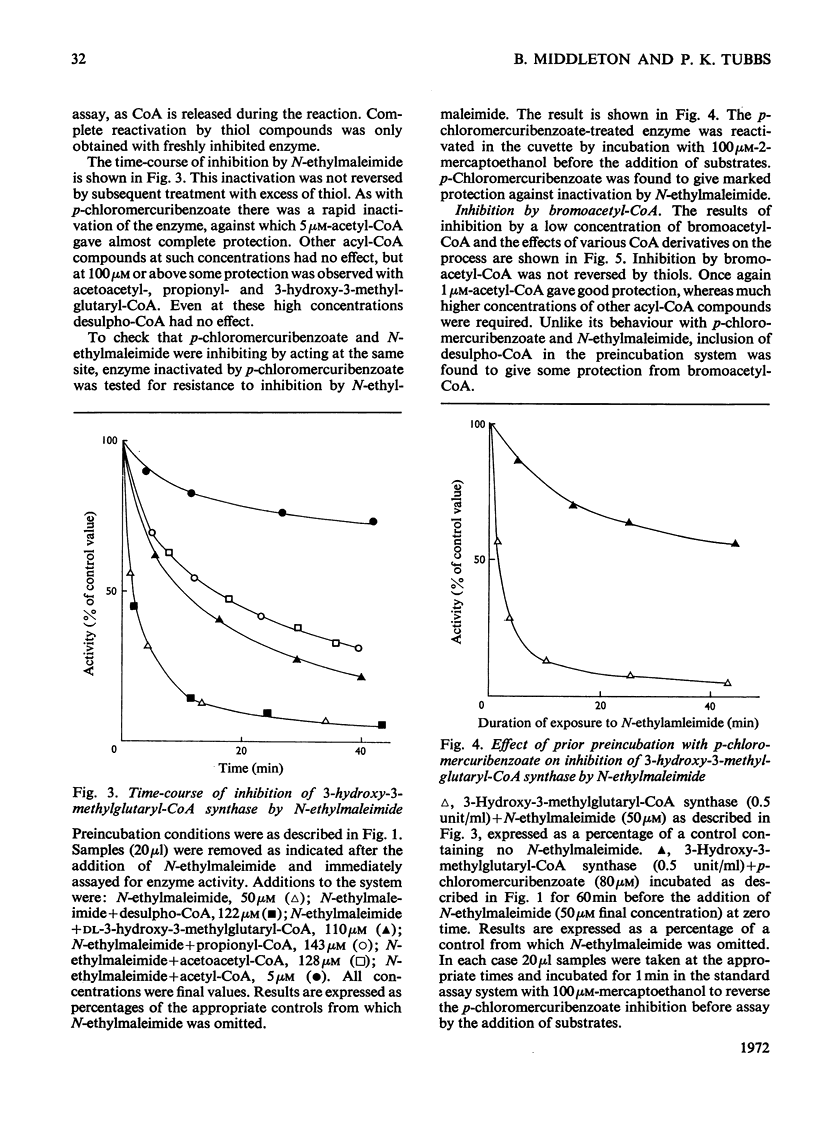
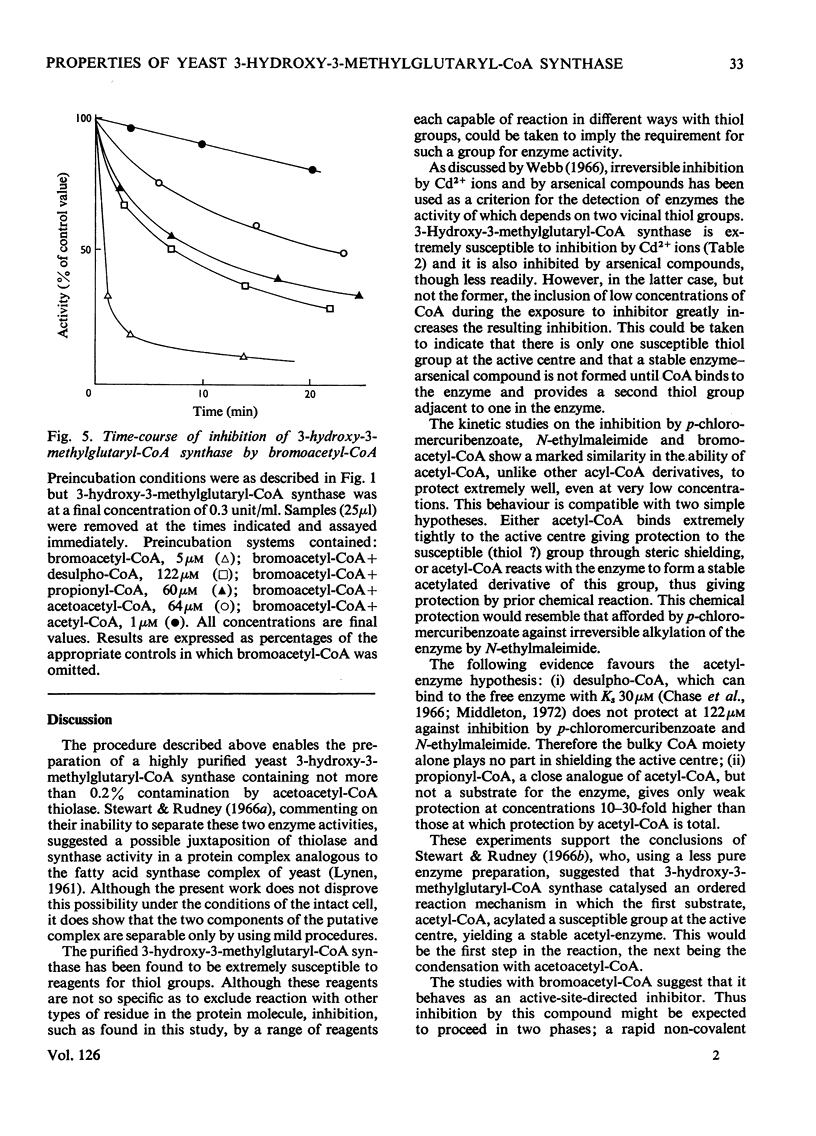
1. A purification of 3-hydroxy-3-methylglutaryl-CoA synthase from baker's yeast is described. This yields a preparation of average specific activity 2.1 units (μmol/min)/mg in which contamination by acetoacetyl-CoA thiolase is less than 0.2%. 2. The molecular weights of 3-hydroxy-3-methylglutaryl-CoA synthase and acetoacetyl-CoA thiolase from baker's yeast were determined by gel filtration on Sephadex G-200. The values obtained were 130000 and 190000 respectively. 3. 3-Hydroxy-3-methylglutaryl-CoA synthase is susceptible to irreversible inhibition by a wide variety of alkylating and acylating agents. The time-course of inhibition of the enzyme by some of these, including the active-site-directed inhibitor bromoacetyl-CoA, was studied in the presence and absence of substrates, products and product analogues. Acetyl-CoA, even when present at concentrations as low as 5μm, gives almost complete protection. Other acyl-CoA derivatives give some protection, but only at concentrations 10–30-fold higher. 4. These results are discussed with reference to an ordered reaction pathway in which acetyl-CoA reacts to give a covalent acetyl-enzyme intermediate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Middleton B., Tubbs P. K. A coenzyme A analogue, desulpho-coA; preparation and effects on various enzymes. Biochem Biophys Res Commun. 1966 Apr 19;23(2):208–213. doi: 10.1016/0006-291x(66)90529-8. [DOI] [PubMed] [Google Scholar]
- Chase J. F. The substrate specificity of carnitine acetyltransferase. Biochem J. 1967 Aug;104(2):510–518. doi: 10.1042/bj1040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F. pH-dependence of carnitine acetyltransferase activity. Biochem J. 1967 Aug;104(2):503–509. doi: 10.1042/bj1040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FERGUSON J. J., Jr, RUDNEY H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. I. Identification and purification of the hydroxymethylglutaryl coenzymecondensing enzyme. J Biol Chem. 1959 May;234(5):1072–1075. [PubMed] [Google Scholar]
- HILZ H., KNAPPE J., RINGELMANN E., LYNEN F. Methylglutaconase, eine neue Hydratase, die am Stoffwechsel verzweigter Carbonsäuren beteiligt ist. Biochem Z. 1958;329(6):476–489. [PubMed] [Google Scholar]
- LYNEN F. Biosynthesis of saturated fatty acids. Fed Proc. 1961 Dec;20:941–951. [PubMed] [Google Scholar]
- LYNEN F. Functional group of coenzyme A and its metabolic relations, especially in the fatty acid cycle. Fed Proc. 1953 Sep;12(3):683–691. [PubMed] [Google Scholar]
- Middleton B., Apps D. K. Subcellular distribution of 3-hydroxy-3-methylglutaryl-CoA synthase, acetoacetyl-CoA thiolase and NAD kinase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1969 Apr 1;177(2):276–285. doi: 10.1016/0304-4165(69)90137-8. [DOI] [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism of 3-hydroxy-3-methylglutaryl-coenzyme A synthase from baker's yeast. Biochem J. 1972 Jan;126(1):35–47. doi: 10.1042/bj1260035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANNER T., PIHL A. The alleged splitting of thioesters by p-chloromercuribenzoate. Biochim Biophys Acta. 1962 Jul 30;62:171–172. doi: 10.1016/0006-3002(62)90505-x. [DOI] [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Stewart P. R., Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. 3. Purification and properties of the condensing enzyme thiolase system. J Biol Chem. 1966 Mar 10;241(5):1212–1221. [PubMed] [Google Scholar]
- Stewart P. R., Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. IV. The origin of the thioester bond of beta-hydroxy-beta-methylglutaryl coenzyme A. J Biol Chem. 1966 Mar 10;241(5):1222–1225. [PubMed] [Google Scholar]


