Abstract
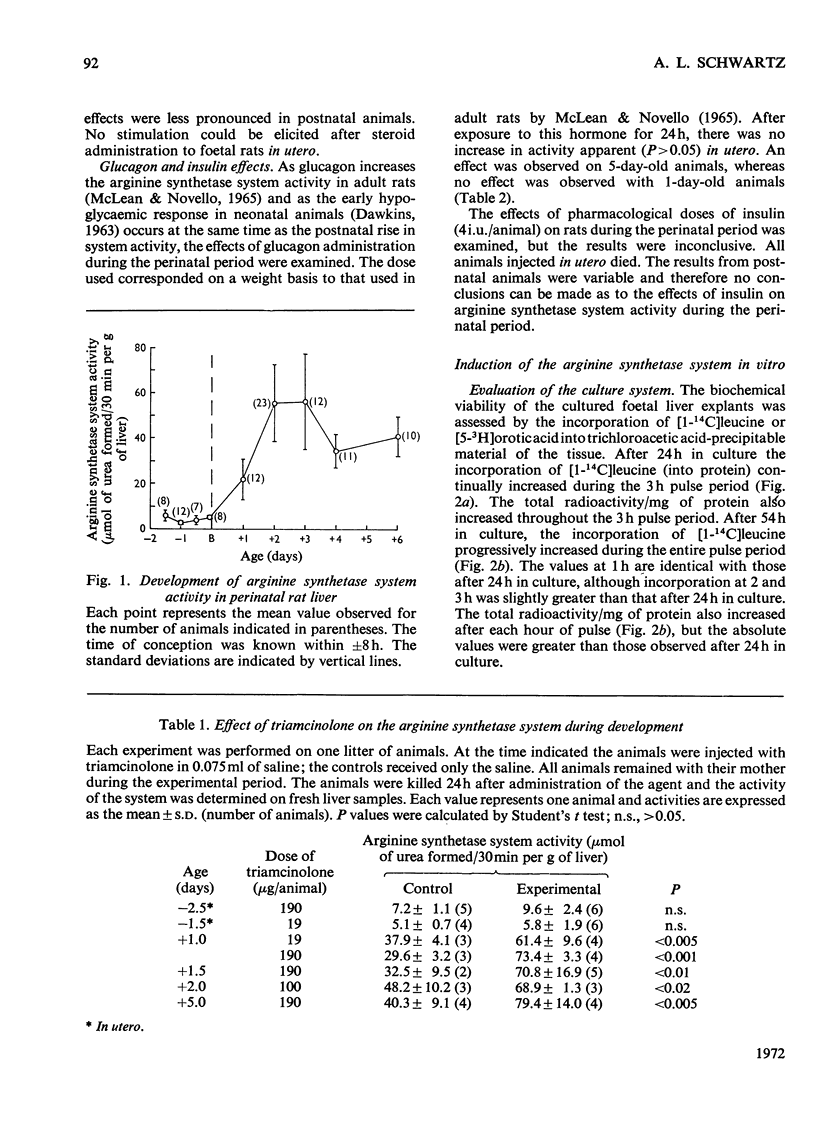
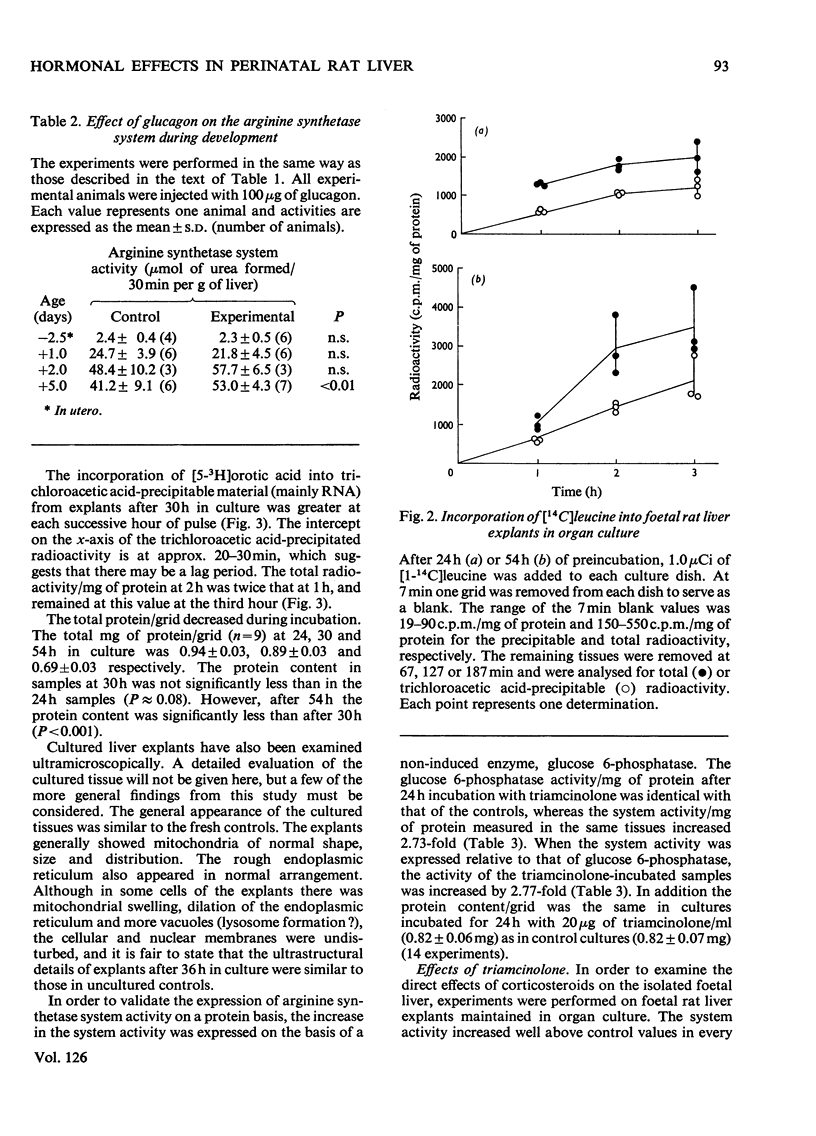
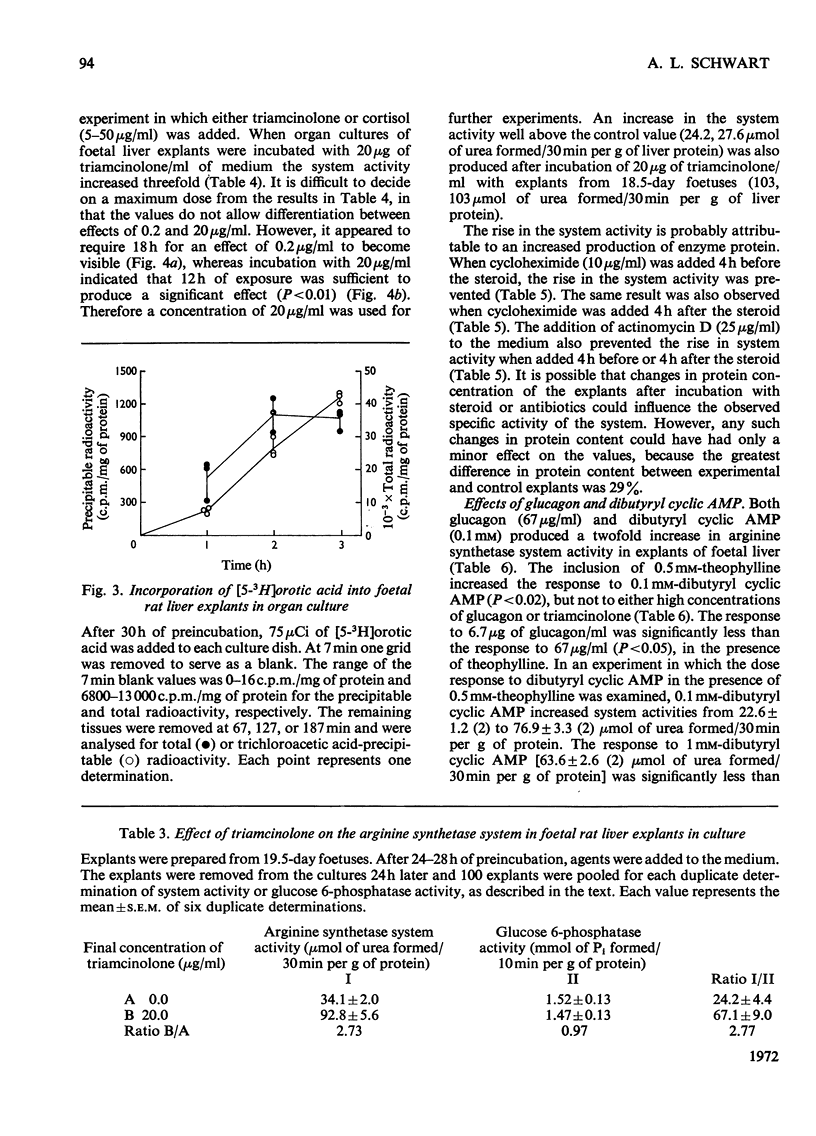
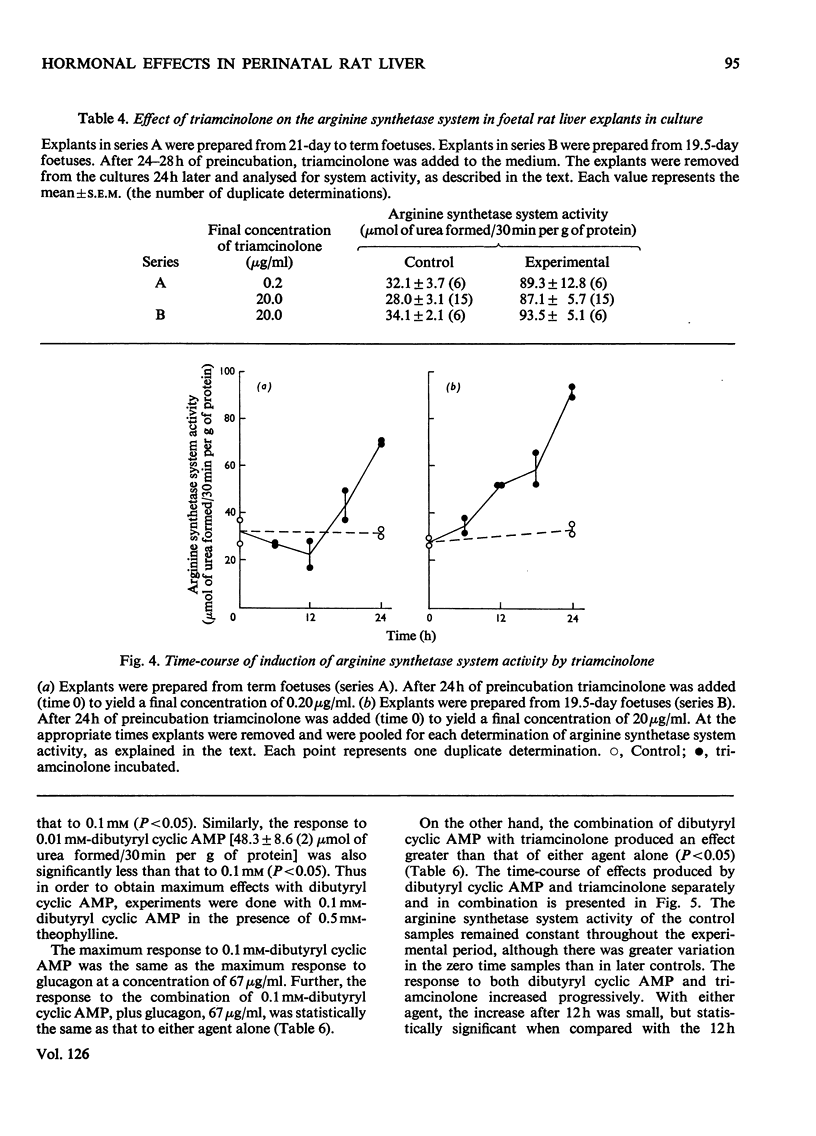
1. The administration of triamcinolone (19–190μg/animal) to postnatal rats increased the arginine synthetase system activity 1.2–2.5-fold above control values 24h after exposure to the hormone. Cortisol (hydrocortisone), however, increased the arginine synthetase system activity only when larger (190μg/animal) or repeated daily doses were given. Glucagon (100μg/animal) stimulated arginine synthetase system activity only after the second postnatal day. None of these agents increased the activity in 19.5–21.5-day foetuses after intrauterine administration. 2. The viability of foetal rat liver explants maintained in organ culture for up to 54h was validated both by ultramicroscopic examination and by incorporation of radioactive leucine and orotic acid. 3. In organ cultures of foetal rat liver explants (18.5 days to term), triamcinolone (20μg/ml of medium) evoked a 2.8–4.3-fold increase after 24h of incubation. This increase was completely inhibited by actinomycin D (25μg/ml) or cycloheximide (10μg/ml). Cortisol (5–50μg/ml) or glucagon (0.067–67μg/ml) also increased the arginine synthetase system activity above the respective control values, but there was no increase in activity with insulin (0.05–0.25i.u./ml). 4. Maximum concentrations of glucagon (67μg/ml), dibutyryl cyclic AMP (6-N,2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate) (0.1mm) and triamcinolone (20μg/ml) incubated for 24h with foetal rat liver explants each produced between a two-and three-fold increase in the activity of the arginine synthetase system. Combinations of maximum amounts of glucagon and the cyclic nucleotide did not produce a greater effect than either agent alone. However, the combination of dibutyryl cyclic AMP with triamcinolone appeared to produce somewhat less than additive effects. 5. The effects of the cyclic nucleotide and triamcinolone were evident after 12h of incubation and increased steadily throughout the 24h of observation. This time-course of increased enzyme activity is very much slower than that reported for the induction of other enzymes in explant cultures of foetal rat liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWKINS M. J. GLYCOGEN SYNTHESIS AND BREAKDOWN IN FETAL AND NEWBORN RAT LIVER. Ann N Y Acad Sci. 1963 Dec 30;111:203–211. doi: 10.1111/j.1749-6632.1963.tb36960.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDLAND R. A., SODIKOFF C. H. Effect of diets and hormones on two urea cycle enzymes. Proc Soc Exp Biol Med. 1962 Feb;109:394–396. doi: 10.3181/00379727-109-27215. [DOI] [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian liver injection of fetal rats with hormones causes the premature formation of liver enzymes. Science. 1969 Feb 28;163(3870):891–895. doi: 10.1126/science.163.3870.891. [DOI] [PubMed] [Google Scholar]
- Greengard O. The hormonal regulation of enzymes in penatal and postnatal rat liver. Effects of adenosine 3',5'-(cyclic)-monophosphate. Biochem J. 1969 Oct;115(1):19–24. doi: 10.1042/bj1150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekomäki M., Schwartz A. L., Pentikäinen P. Rate of urea synthesis in normal and cirrhotic rat liver with reference to the arginine synthetase system. Scand J Gastroenterol. 1970;5(5):375–380. [PubMed] [Google Scholar]
- Kekomäki M., Seppälä M., Ehnholm C., Schwartz A. L., Raivio K. Perfusion of isolated human fetal liver: synthesis and release of -fetoprotein and albumin. Int J Cancer. 1971 Sep 15;8(2):250–258. doi: 10.1002/ijc.2910080209. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCLEAN P., GURNEY M. W. Effect of adrenalectomy and of growth hormone on enzymes concerned with urea synthesis in rat liver. Biochem J. 1963 Apr;87:96–104. doi: 10.1042/bj0870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P., NOVELLO F. INFLUENCE OF PANCREATIC HORMONES ON ENZYMES CONCERNED WITH UREA SYNTHESIS IN RAT LIVER. Biochem J. 1965 Feb;94:410–422. doi: 10.1042/bj0940410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räihä N. C., Suihkonen J. Factors influencing the development of urea-synthesizing enzymes in rat liver. Biochem J. 1968 May;107(6):793–797. doi: 10.1042/bj1070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. Studies on factors affecting the levels of urea cycle enzymes in rat liver. J Biol Chem. 1963 Mar;238:1012–1018. [PubMed] [Google Scholar]
- TRUMP B. F., GOLDBLATT P. J., STOWELL R. E. STUDIES ON NECROSIS OF MOUSE LIVER IN VITRO. ULTRASTRUCTURAL ALTERATIONS IN THE MITOCHONDRIA OF HEPATIC PARENCHYMAL CELLS. Lab Invest. 1965 Apr;14:343–371. [PubMed] [Google Scholar]
- Wicks W. D. Induction of hepatic enzymes by adenosine 3',5'-monophosphate in organ culture. J Biol Chem. 1969 Jul 25;244(14):3941–3950. [PubMed] [Google Scholar]
- Wicks W. D. Induction of tyrosine-alpha-ketoglutarate transaminase in fetal rat liver. J Biol Chem. 1968 Mar 10;243(5):900–906. [PubMed] [Google Scholar]


