Abstract
1. Lipogenesis in sheep liver and adipose tissue was investigated by incorporation studies in vitro with radioactive glucose and acetate and by assays of key enzymes. 2. Carbohydrate availability to sheep was increased by feeding on a diet containing 70% soluble carbohydrate, by infusing glucose into the abomasum or by direct intravenous infusion of glucose. 3. Under these conditions lipogenesis from glucose and acetate was increased from very low values in lìver and adipose tissue, especially in those animals where rumen fermentation was by-passed by glucose infusion. 4. Large increases in the activities of ATP citrate lyase (EC 4.1.3.8) and NADP–malate dehydrogenase (EC 1.1.1.40) occurred in both tissues when lipogenesis was increased. 5. No adaptations were found in the activities of pyruvate carboxylase (EC 6.4.1.1) in adipose tissue, glucokinase (EC 2.7.1.2) in liver or 3-hydroxybutyrate dehydrogenase (EC 1.1.1.30) in liver. It is proposed that the absence of these enzymes is not related to glucose availability. 6. The effect of glucose on liver lipogenesis was to increase conversion of acetate into lipid. 7. This effect also occurred in adipose tissue, but in this tissue glucose also became a quantitatively important precursor of triglyceride fatty acid.
Full text
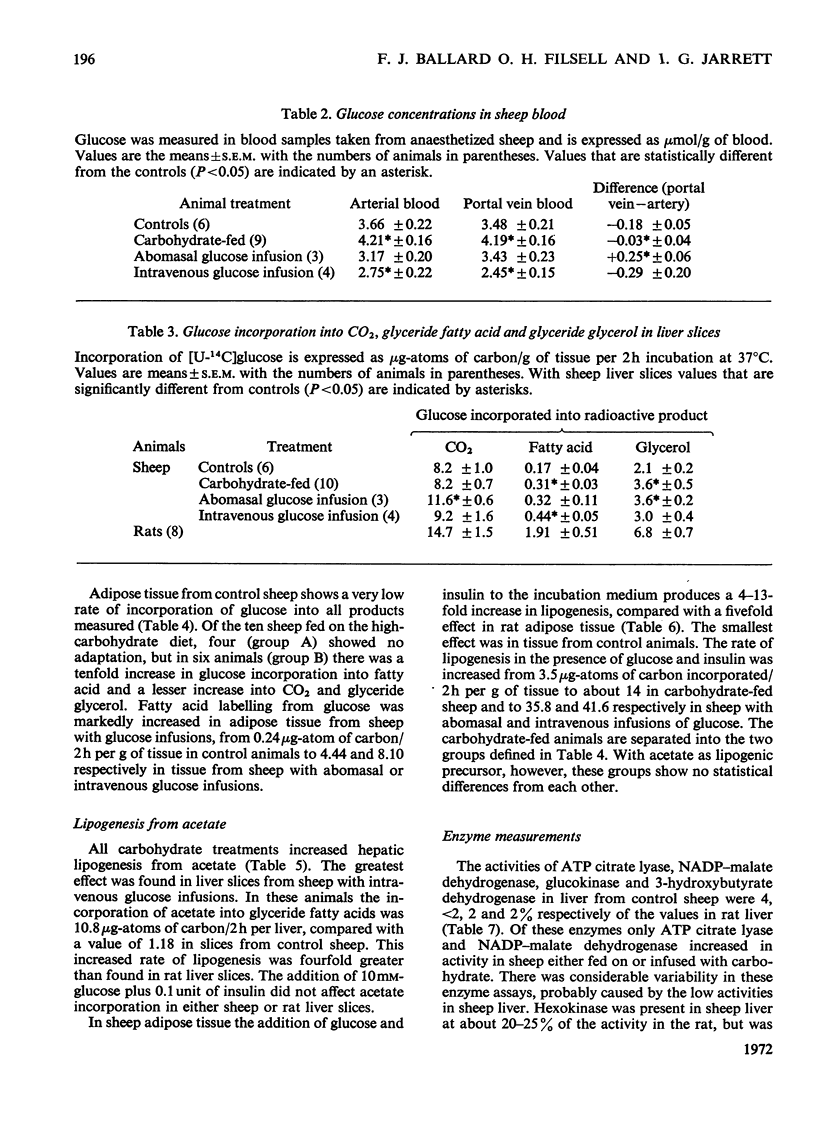
PDF


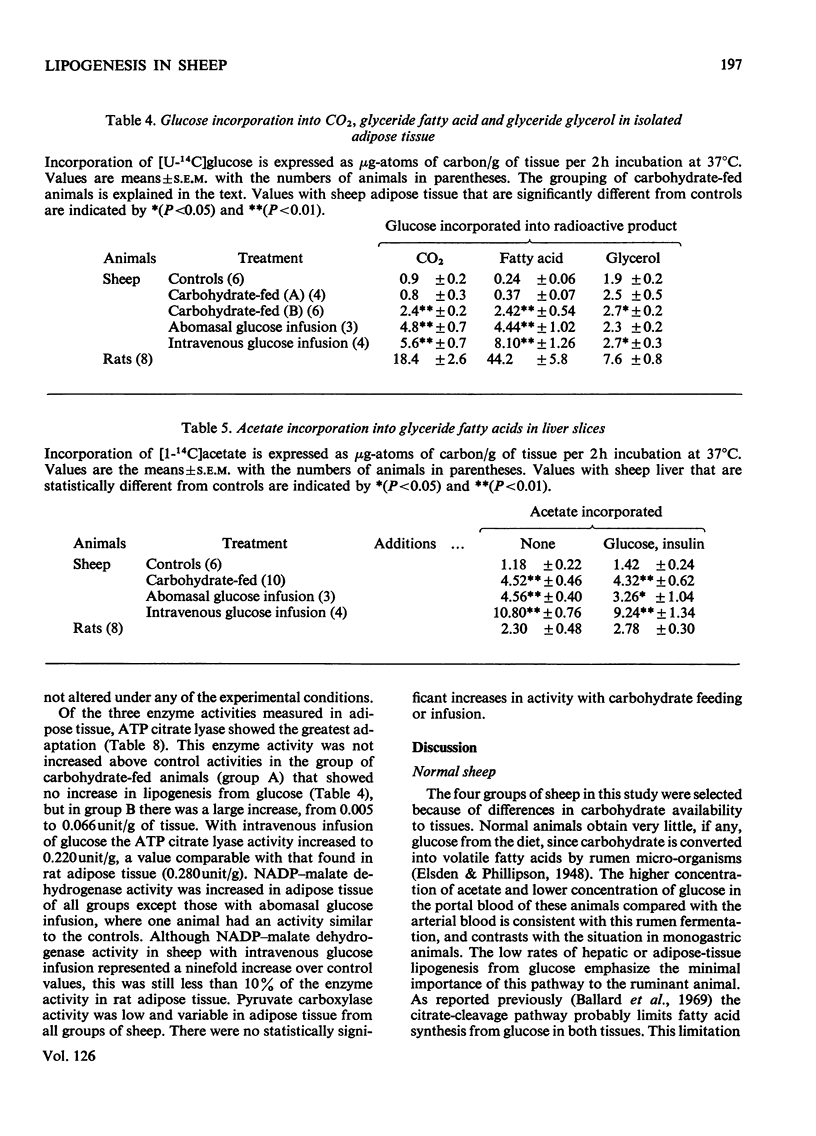
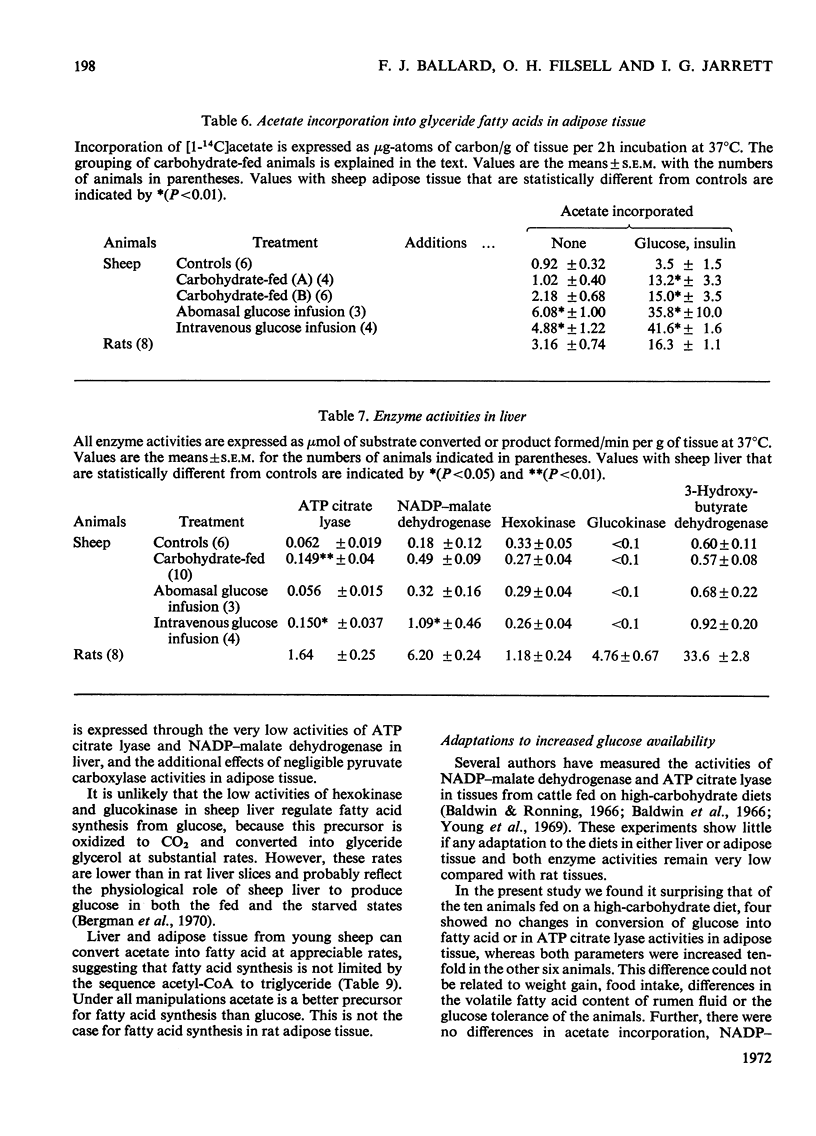
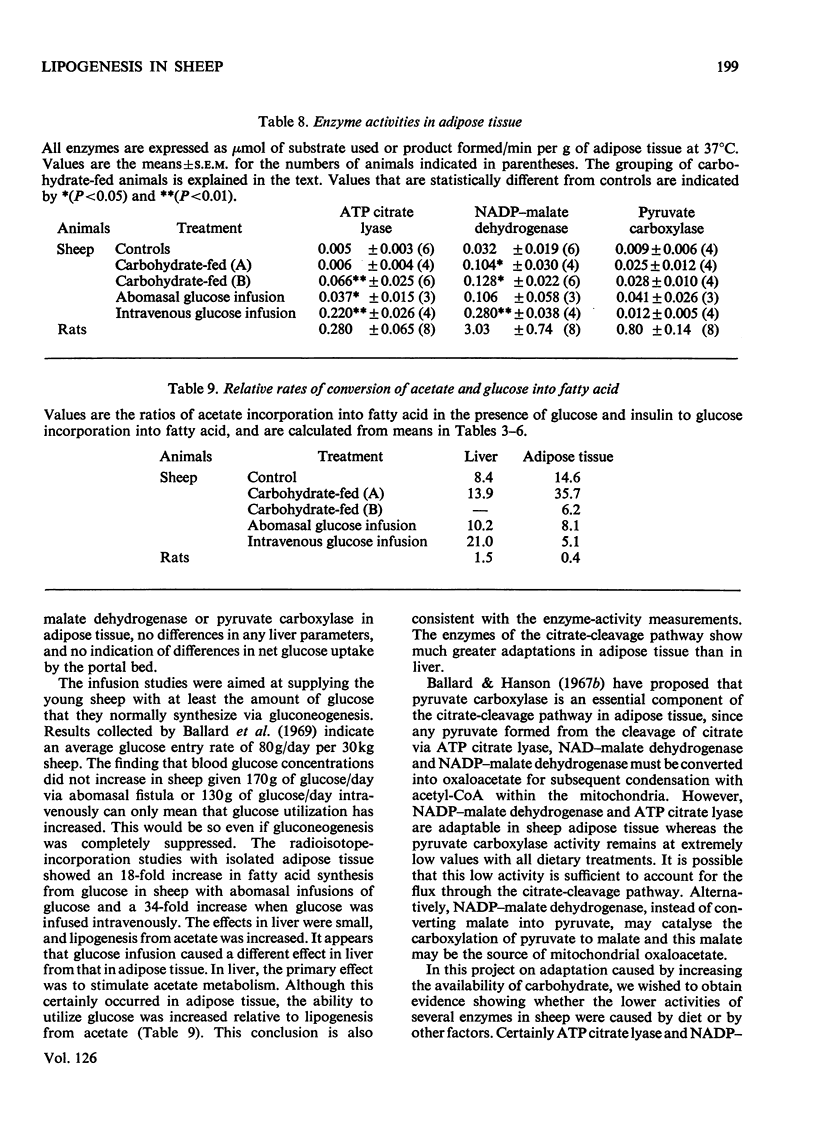

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson R. W., Ballard F. J. The metabolic fate of the products of citrate cleavage. Adenosine triphosphate-citrate lyase and nicotinamide-adenine dinucleotide phosphate-linked malate dehydrogenase in foetal and adult liver from ruminants and non-ruminants. Biochem J. 1968 Aug;108(5):705–713. doi: 10.1042/bj1080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Ronning M. Dietary fat levels and carbohydrate and fat metabolism in calves. J Dairy Sci. 1966 Jun;49(6):688–689. doi: 10.3168/jds.S0022-0302(66)87933-X. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L., Ronning M., Radanovics C., Plange G. Effect of carbohydrate and fat intakes upon the activities of several liver enzymes in rats, guinea piglets, piglets and calves. J Nutr. 1966 Sep;90(1):47–55. doi: 10.1093/jn/90.1.47. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Changes in lipid synthesis in rat liver during development. Biochem J. 1967 Mar;102(3):952–958. doi: 10.1042/bj1020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W., Kronfeld D. S. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed Proc. 1969 Jan-Feb;28(1):218–231. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. The citrate cleavage pathway and lipogenesis in rat adipose tissue: replenishment of oxaloacetate. J Lipid Res. 1967 Mar;8(2):73–79. [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. Ketohexokinase, isoenzymes of glucokinase and glycogen synthesis from hexoses in neonatal rat liver. Biochem J. 1964 Feb;90(2):261–268. doi: 10.1042/bj0900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Oliver I. T. The effect of concentration on glucose phosphorylation and incorporation into glycogen in the livers of foetal and adult rats and sheep. Biochem J. 1964 Jul;92(1):131–136. doi: 10.1042/bj0920131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman E. N., Katz M. L., Kaufman C. F. Quantitative aspects of hepatic and portal glucose metabolism and turnover in sheep. Am J Physiol. 1970 Sep;219(3):785–793. doi: 10.1152/ajplegacy.1970.219.3.785. [DOI] [PubMed] [Google Scholar]
- Cramp D. G. New automated method for measuring glucose by glucose oxidase. J Clin Pathol. 1967 Nov;20(6):910–912. doi: 10.1136/jcp.20.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J. 1967 Nov;105(2):529–536. doi: 10.1042/bj1050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. C. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochem J. 1966 Apr;99(1):228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- Muramatsu M., Ambo K., Tsuda T. ATP citrate lyase activity in the liver of newborn lambs. J Biochem. 1970 May;67(5):727–729. doi: 10.1093/oxfordjournals.jbchem.a129299. [DOI] [PubMed] [Google Scholar]
- SCHWARZ K. Production of dietary necrotic liver degeneration using American torula yeast. Proc Soc Exp Biol Med. 1951 Aug;77(4):818–823. doi: 10.3181/00379727-77-18935. [DOI] [PubMed] [Google Scholar]
- Young J. W., Thorp S. L., De Lumen H. Z. Activity of selected gluconeogenic and lipogenic enzymes in bovine rumen mucosa, liver and adipose tissue. Biochem J. 1969 Aug;114(1):83–88. doi: 10.1042/bj1140083. [DOI] [PMC free article] [PubMed] [Google Scholar]


