Abstract
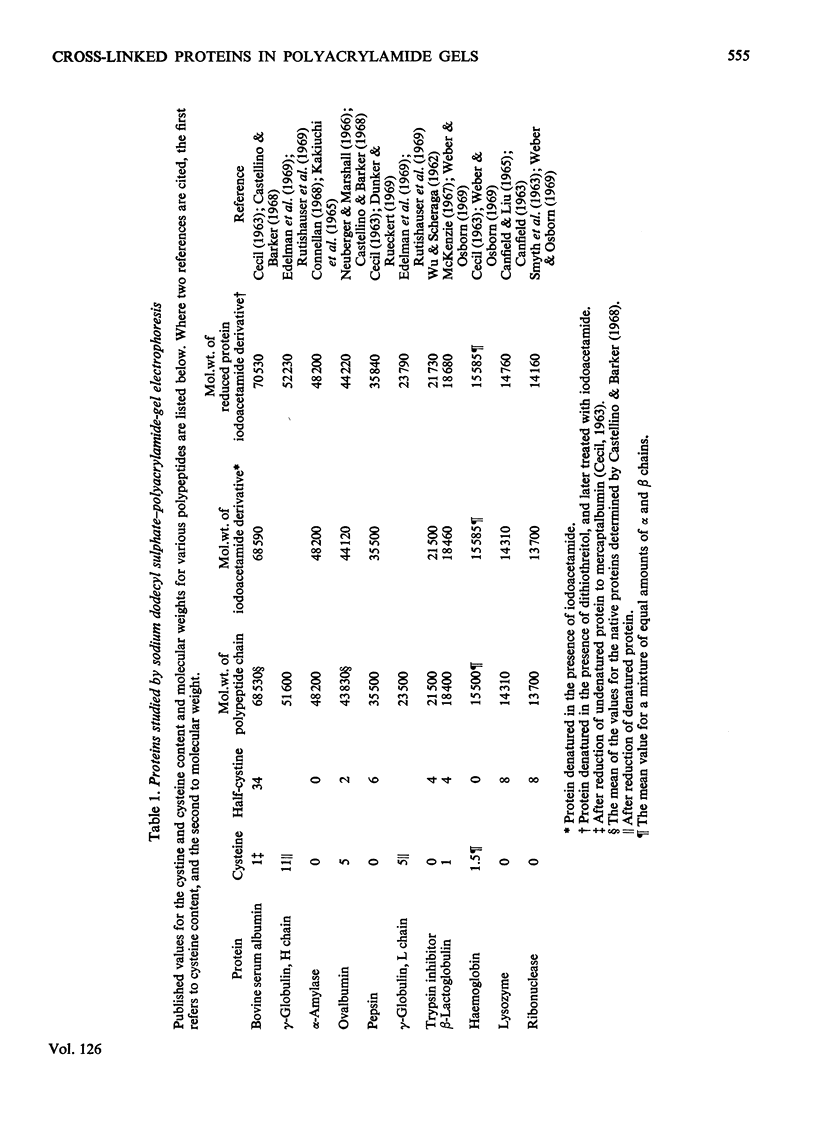
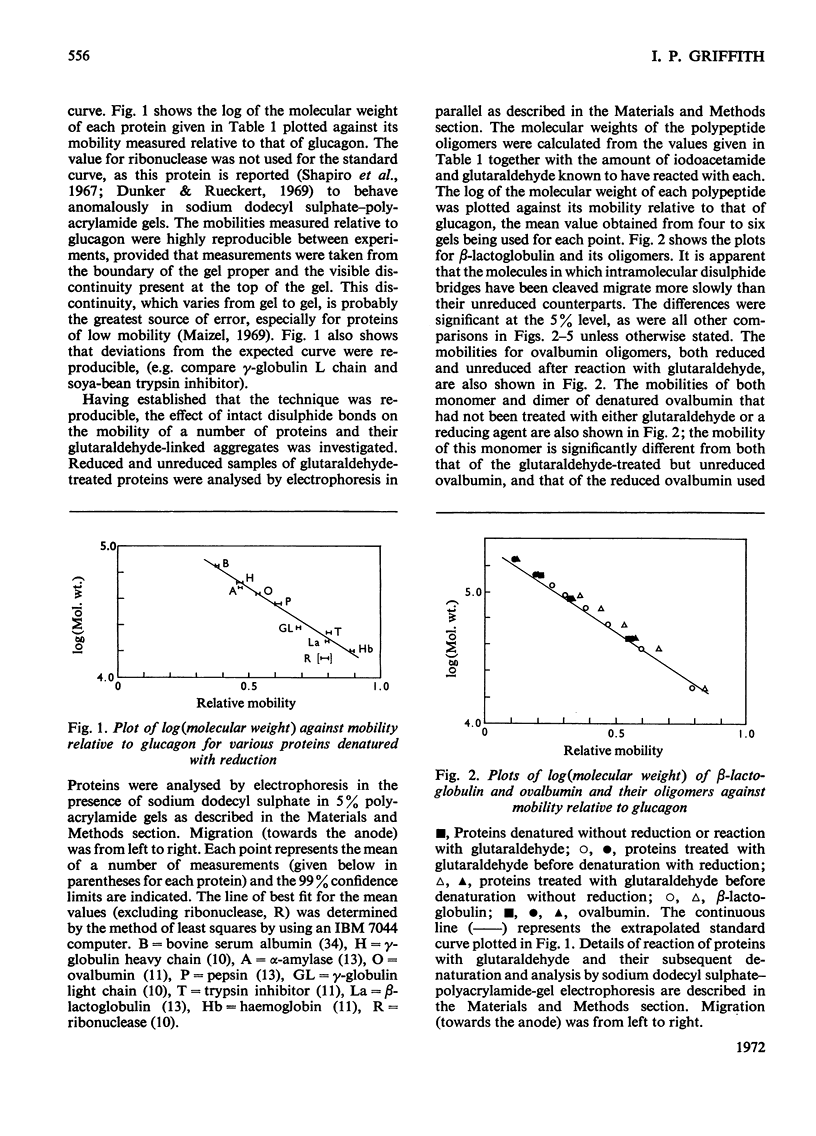
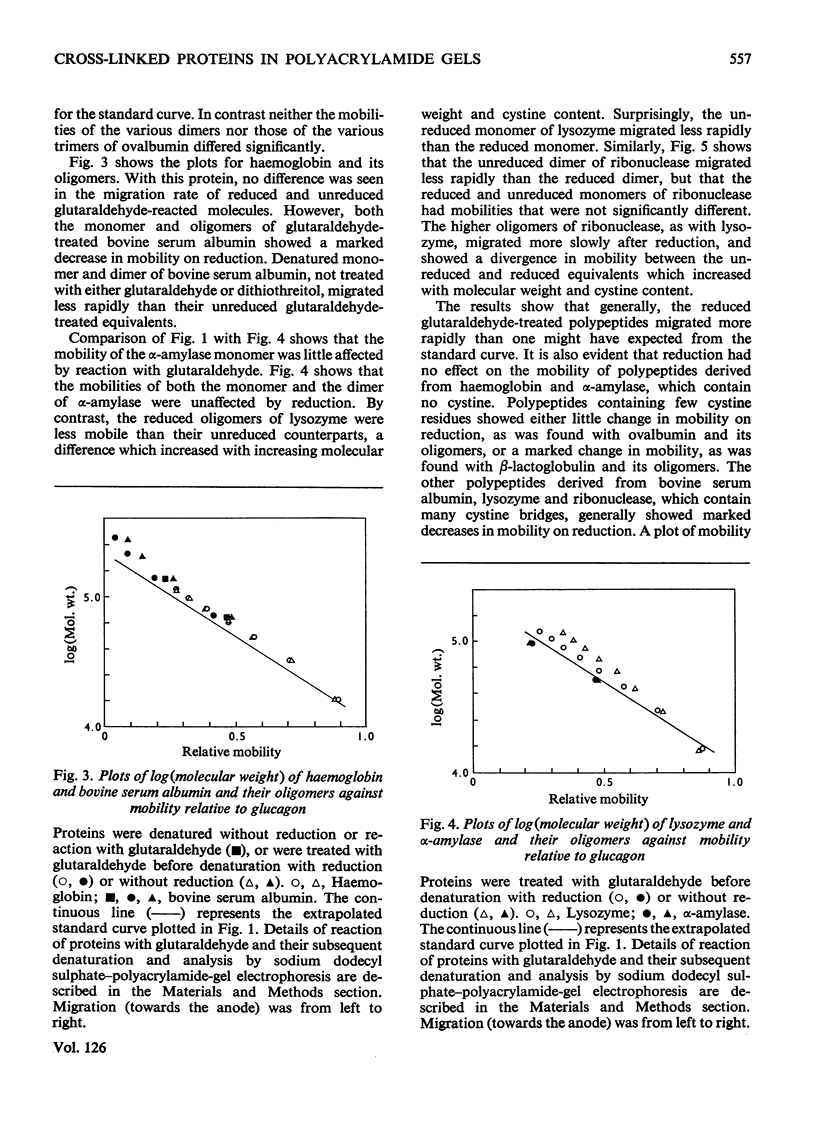
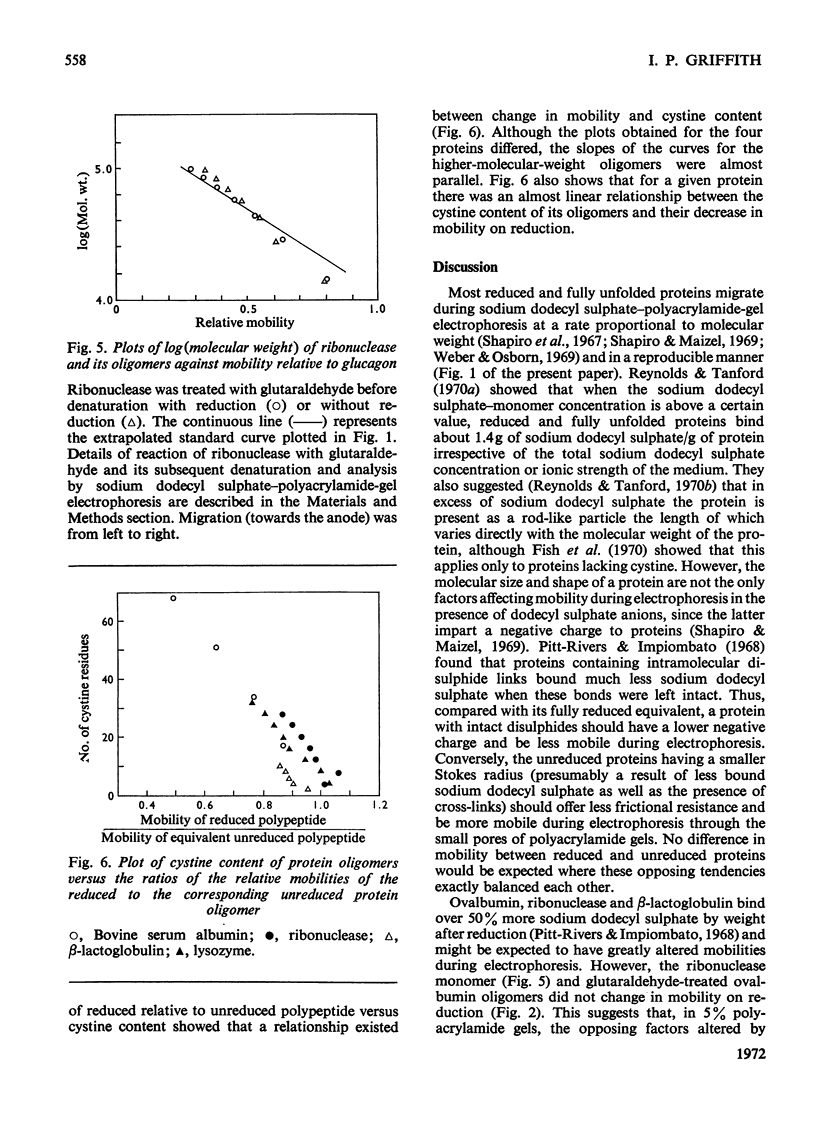
The effect of reduction of intramolecular disulphide bridges on the mobility of proteins in 5% (w/v) polyacrylamide gels in the presence of sodium dodecyl sulphate was investigated. A series of polypeptide polymers, containing up to 68 intramolecular disulphide bridges, was prepared by cross-linking proteins of known structure with glutaraldehyde. These model polypeptides were denatured with heat, sodium dodecyl sulphate and urea, and their mobilities in sodium dodecyl sulphate–polyacrylamide gels compared before and after reduction with dithiothreitol. The mobilities of polypeptides containing no cystine were unaffected by reduction. However, reduction generally decreased the mobilities of polypeptides containing cystine; the extent of this decrease depended on the number of cystine residues originally present in the polypeptide polymer, and on the protein from which the latter was derived. In contrast with their higher oligomers, the monomer of lysozyme and the dimer of ribonuclease increased in mobility after reduction. The reduced polypeptide oligomers formed by reaction with glutaraldehyde were generally found to migrate at a rate significantly faster than was expected from their calculated molecular weights. It was concluded that the use of unreduced proteins and protein aggregates for molecular-weight measurements by the sodium dodecyl sulphate–polyacrylamide-gel method may give erroneous estimates of the molecular weight of any protein being investigated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- KAKIUCHI K., HAMAGUCHI K., ISEMURA T. ASSOCIATION AND DISSOCIATION OF BACILLUS SUBTILIS ALPHA-AMYLASE MOLECULE. 3. THE EFFECTS OF PH AND SALTS ON THE MONOMER-DIMER TRANSFORMATION. J Biochem. 1965 Feb;57:167–175. doi: 10.1093/oxfordjournals.jbchem.a128073. [DOI] [PubMed] [Google Scholar]
- McKenzie H. A. Milk proteins. Adv Protein Chem. 1967;22:55–234. doi: 10.1016/s0065-3233(08)60041-8. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Rutishauser U., Cunningham B. A., Bennett C., Konigsberg W. H., Edelman G. M. Amino acid sequence of the Fc region of a human gamma G-immunoglobulin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1414–1421. doi: 10.1073/pnas.61.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Knight C. A. Effect of charge on the determination of molecular weight of proteins by gel electrophoresis in SDS. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1117–1121. doi: 10.1016/0006-291x(71)90020-9. [DOI] [PubMed] [Google Scholar]
- WU Y. V., SCHERAGA H. A. Studies of soybean trypsin inhibitor. I. Physicochemical properties. Biochemistry. 1962 Jul;1:698–705. doi: 10.1021/bi00910a025. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Tu J. I. Modification of glycogen phosphorylase b by glutaraldehyde. Preparation and isolation of enzyme derivatives with enhanced stability. Biochemistry. 1969 Nov;8(11):4403–4410. doi: 10.1021/bi00839a027. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolf B., Lausarot P. M., Lesnaw J. A., Reichmann M. E. Preparation of polymeric protein markers and an investigation of their behavior in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Jan 20;200(1):180–183. doi: 10.1016/0005-2795(70)90060-7. [DOI] [PubMed] [Google Scholar]


