Abstract
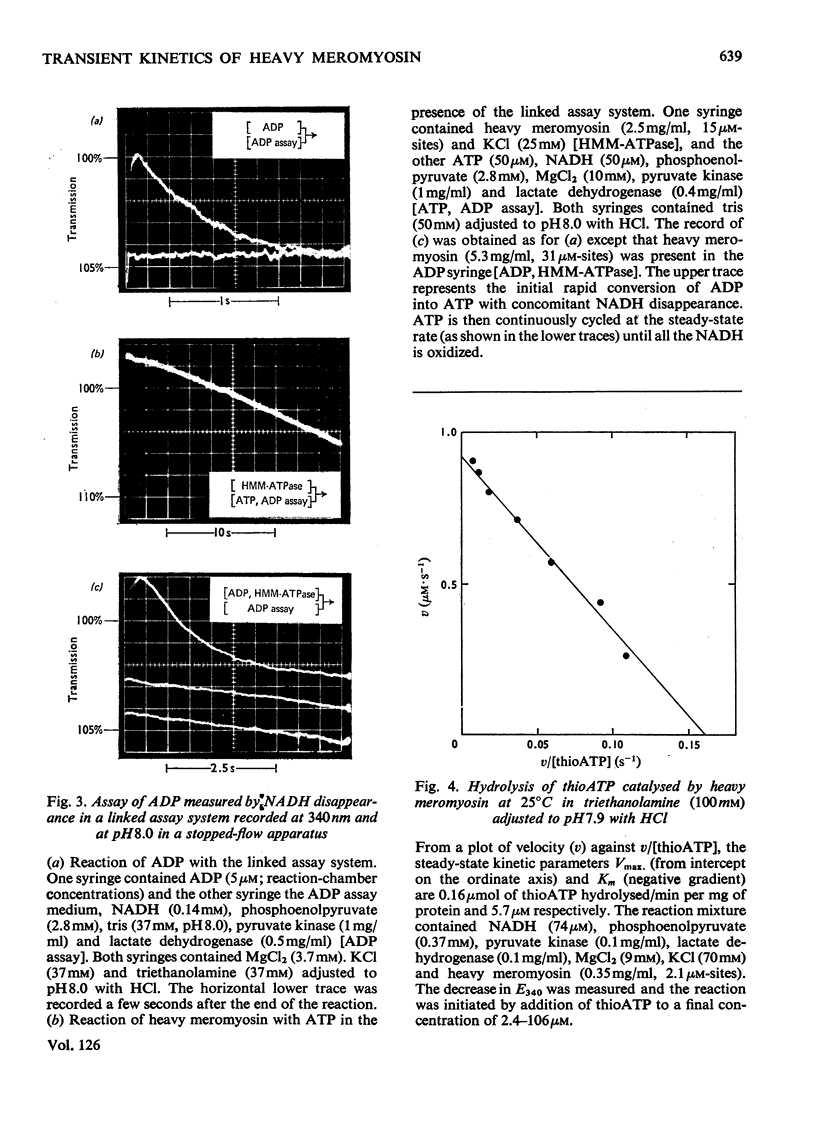
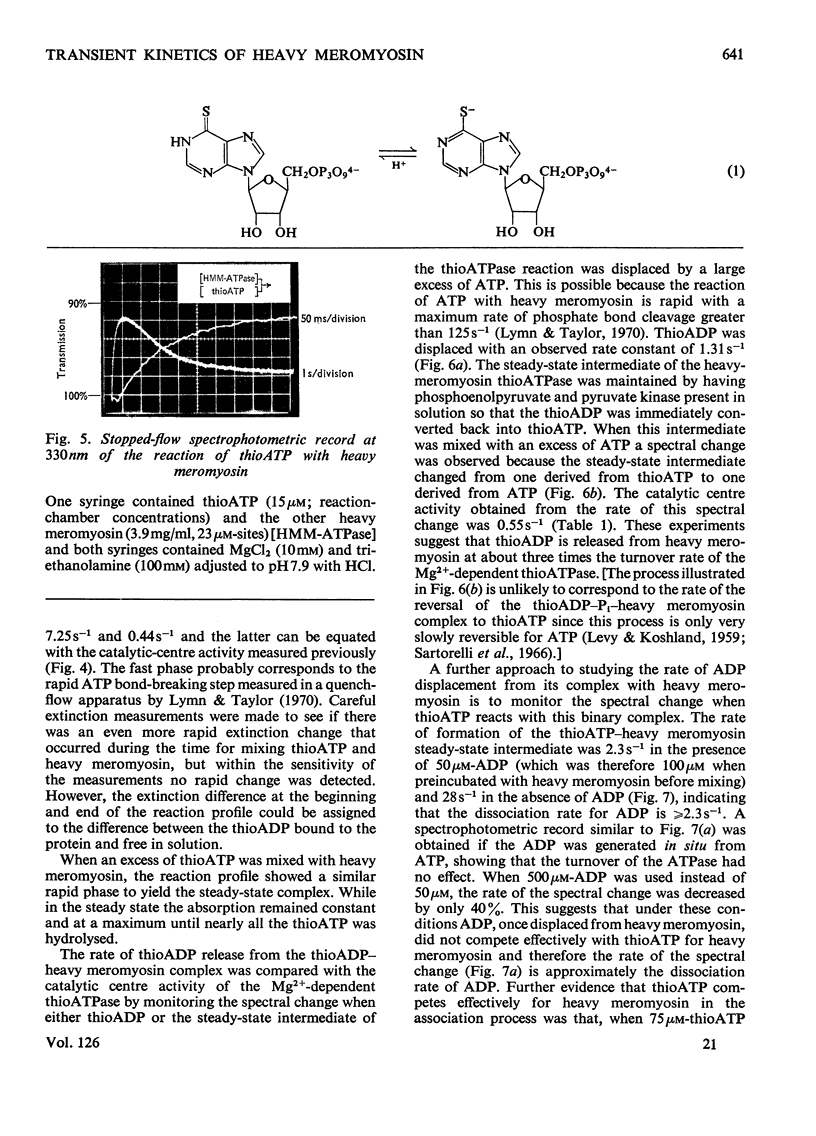
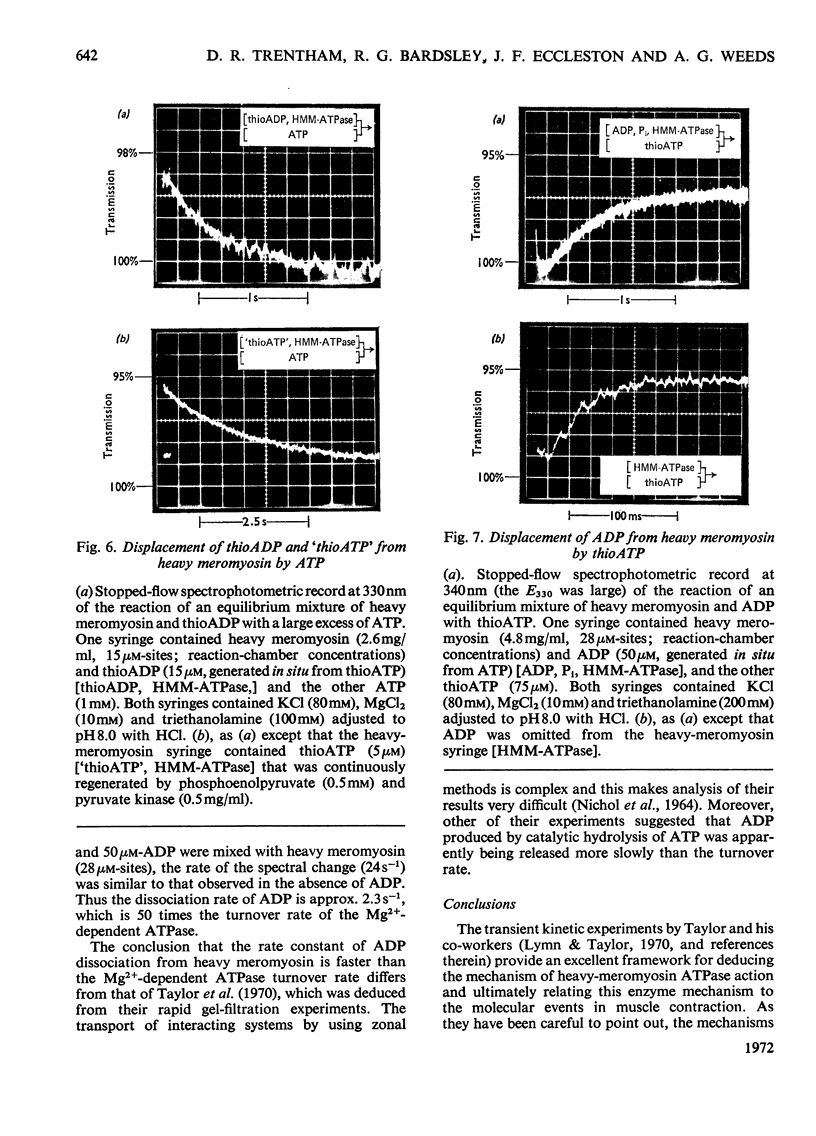
Transient kinetic studies of Mg2+-dependent heavy-meromyosin ATPase (adenosine triphosphatase) were done by monitoring the release of both ADP and Pi into the reaction medium by using linked assay systems. The release of Pi was monitored by its quantitative transfer to ADP, with concomitant reduction of NAD+ in the presence of d-glyceraldehyde 3-phosphate, d-glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase. The dissociation rates of the products, ADP and Pi, from heavy meromyosin were shown to be faster than the rate-controlling process, which occurs after the initial bond cleavage of ATP. The chromophoric ATP analogue, 6-mercapto-9-β-d-ribofuranosylpurine 5′-triphosphate (thioATP) was used as a substrate and spectral changes associated with a single turnover of heavy meromyosin could be assigned to elementary processes of the mechanism. It was shown that the dissociation rate of thioADP was not the rate-controlling process of the thioATPase, whose catalytic-centre activity was 7.6 times that of the ATPase at pH8. The dissociation rate of ADP from heavy meromyosin was measured by using thioATP as displacing agent and was found to be 2.3s−1, which is about 50 times the catalytic-centre activity of the ATPase at pH8. Transient kinetic studies with chromophoric adenosine phosphate analogues have general application for kinases and ATPases both in characterizing the chemical states of the intermediates and in delineating the elementary processes of the enzyme mechanism.
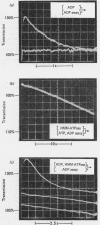
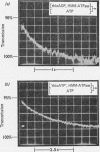
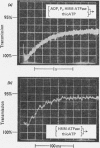
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS S. A., LAWRENCE J. C., RICKETTS C. R. The phosphate esters of mammalian skin maintained on glucose and various deoxyglucoses. Biochem J. 1959 Dec;73:566–572. doi: 10.1042/bj0730566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman T. E., Gutfreund H. Optical and chemical identification of kinetic steps in trypsin- and chymotrypsin-catalysed reactions. Biochem J. 1966 Nov;101(2):411–416. doi: 10.1042/bj1010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. J Biol Chem. 1970 May 10;245(9):2451–2456. [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Gutfreund H. Transients and relaxation kinetics of enzyme reactions. Annu Rev Biochem. 1971;40:315–344. doi: 10.1146/annurev.bi.40.070171.001531. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Imamura K., Tada M., Tonomura Y. The pre-steady state of the myosin--adenosine triphosphate system. IV. Liberation of ADP from the myosin--ATP system and effects of modifiers on the phosphorylation of myosin. J Biochem. 1966 Mar;59(3):280–289. [PubMed] [Google Scholar]
- LEVY H. M., KOSHLAND D. E., Jr Mechanism of hydrolysis of adenosinetriphosphate by muscle proteins and its relation to muscular contraction. J Biol Chem. 1959 May;234(5):1102–1107. [PubMed] [Google Scholar]
- Lowey S., Luck S. M. Equilibrium binding of adenosine diphosphate to myosin. Biochemistry. 1969 Aug;8(8):3195–3199. doi: 10.1021/bi00836a010. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- Morita F. Interaction of heavy meromyosin with substrate. I. Difference in ultraviolet absorption spectrum between heavy meromyosin and its Michaelis-Menten complex. J Biol Chem. 1967 Oct 10;242(19):4501–4506. [PubMed] [Google Scholar]
- Murphy A. J., Duke J. A., Stowring L. Synthesis of 6-mercapto-9-beta-D-ribofuranosylpurine 5'-triphosphate, a sulfhydryl analog of ATP. Arch Biochem Biophys. 1970 Mar;137(1):297–298. doi: 10.1016/0003-9861(70)90441-8. [DOI] [PubMed] [Google Scholar]
- Murphy A. J., Morales M. F. Number and location of adenosine triphosphatase sites of myosin. Biochemistry. 1970 Mar 31;9(7):1528–1532. doi: 10.1021/bi00809a008. [DOI] [PubMed] [Google Scholar]
- SZEWCZUK A., WOLNY E., WOLNY M., BARANOWSKI T. [A new method for obtaining d-glyceraldehyde-3-phosphate]. Acta Biochim Pol. 1961;8:201–207. [PubMed] [Google Scholar]
- Sartorelli L., Fromm H. J., Benson R. W., Boyer P. D. Direct and 18-O-exchange measurements relevant to possible activated or phosphorylated states of myosin. Biochemistry. 1966 Sep;5(9):2877–2884. doi: 10.1021/bi00873a015. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. An improved procedure for the isolation of 3-phosphoglycerate kinase from yeast. Biochem J. 1971 Mar;122(1):89–92. doi: 10.1042/bj1220089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stinson R. A., Gutfreund H. Transient-kinetic studies of pig muscle lactate dehydrogenase. Biochem J. 1971 Jan;121(2):235–240. doi: 10.1042/bj1210235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. R., Yount R. G. A manganese stimulated, nucleotide dependent 18-O-inorganic phosphate exchange reaction catalyzed by heavy meromyosin. Biochem Biophys Res Commun. 1965 Jun 9;19(6):765–769. doi: 10.1016/0006-291x(65)90325-6. [DOI] [PubMed] [Google Scholar]
- Taylor E. W., Lymn R. W., Moll G. Myosin-product complex and its effect on the steady-state rate of nucleoside triphosphate hydrolysis. Biochemistry. 1970 Jul 21;9(15):2984–2991. doi: 10.1021/bi00817a008. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Morales M. F. Independent and cooperative reactions of myosin heads with F-actin in the presence of adenosine triphosphate. Biochemistry. 1971 Apr 27;10(9):1722–1727. doi: 10.1021/bi00785a033. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Rate-determining processes and the number of simultaneously active sties of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1971 Mar;122(1):71–77. doi: 10.1042/bj1220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Reactions of D-glyceraldehyde 3-phosphate dehydrogenase facilitated by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1971 Mar;122(1):59–69. doi: 10.1042/bj1220059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. C., Cerami A., Reich E., Acs G., Altwerger L. Biochemical studies of the nucleoside analogue, formycin. J Biol Chem. 1969 Jun 25;244(12):3243–3250. [PubMed] [Google Scholar]
- Ward D. C., Reich E., Stryer L. Fluorescence studies of nucleotides and polynucleotides. I. Formycin, 2-aminopurine riboside, 2,6-diaminopurine riboside, and their derivatives. J Biol Chem. 1969 Mar 10;244(5):1228–1237. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]