Abstract
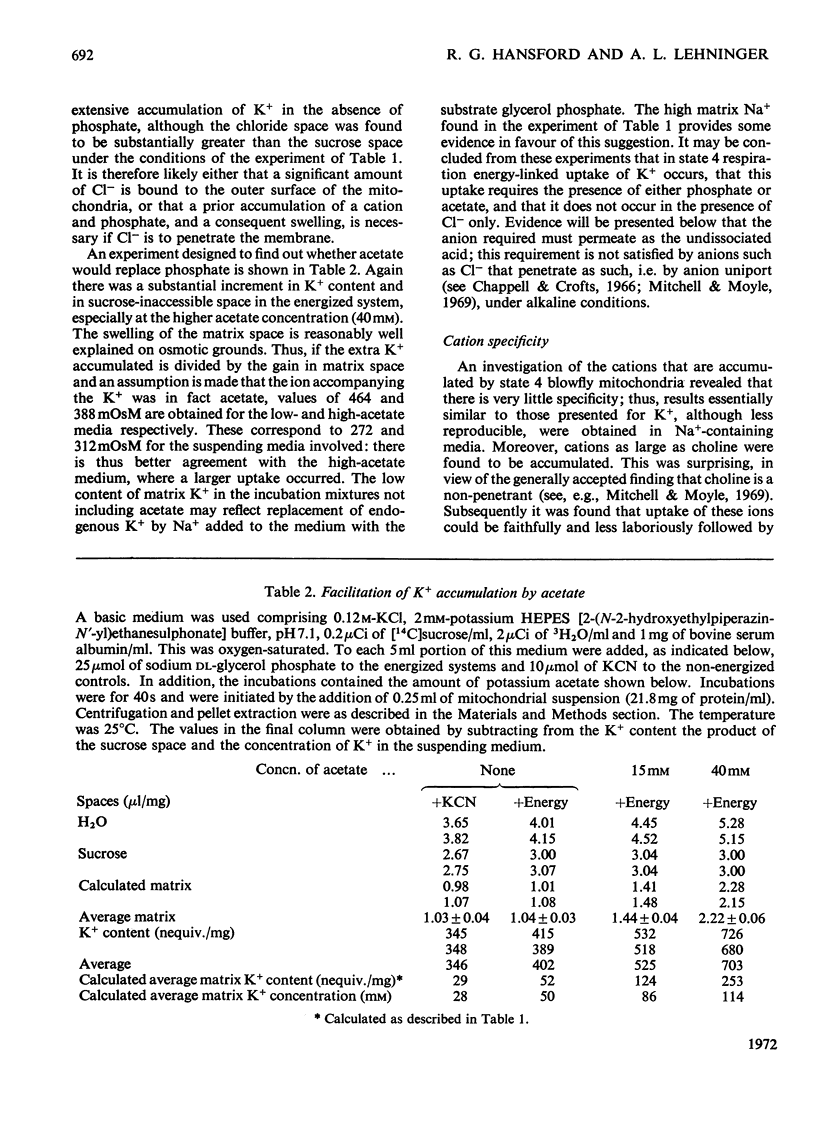
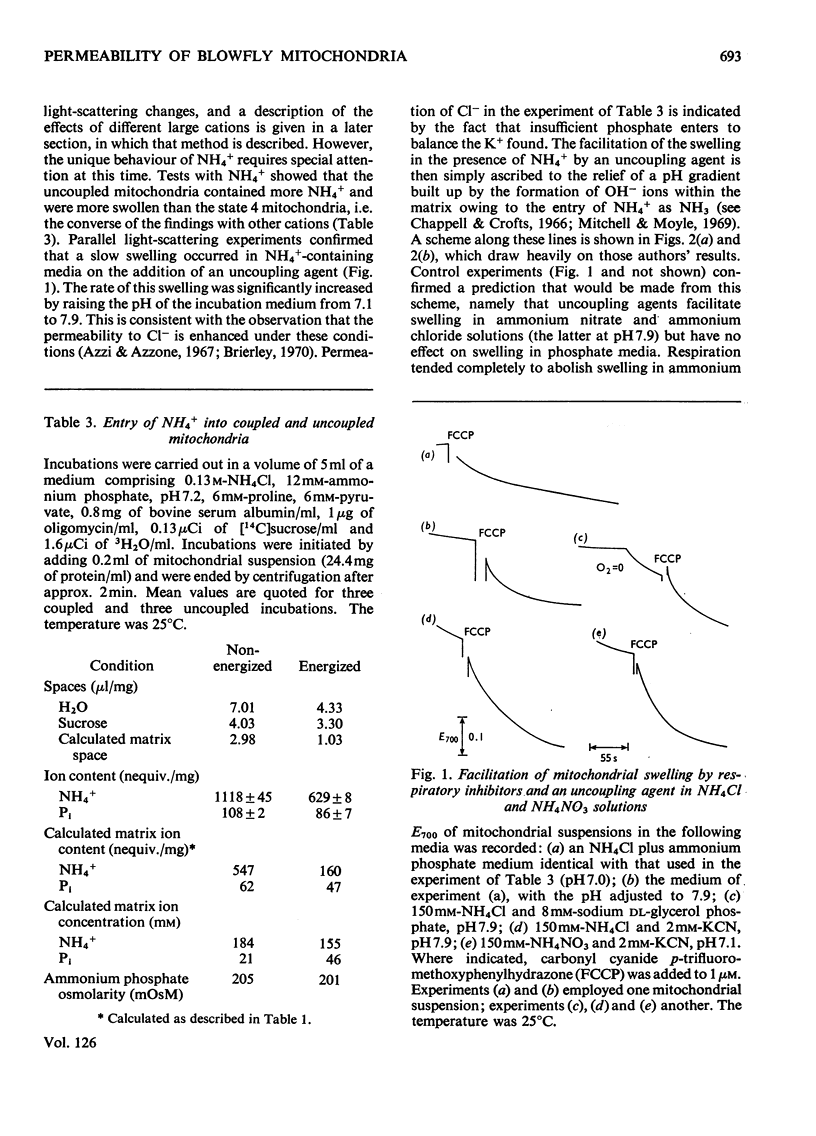
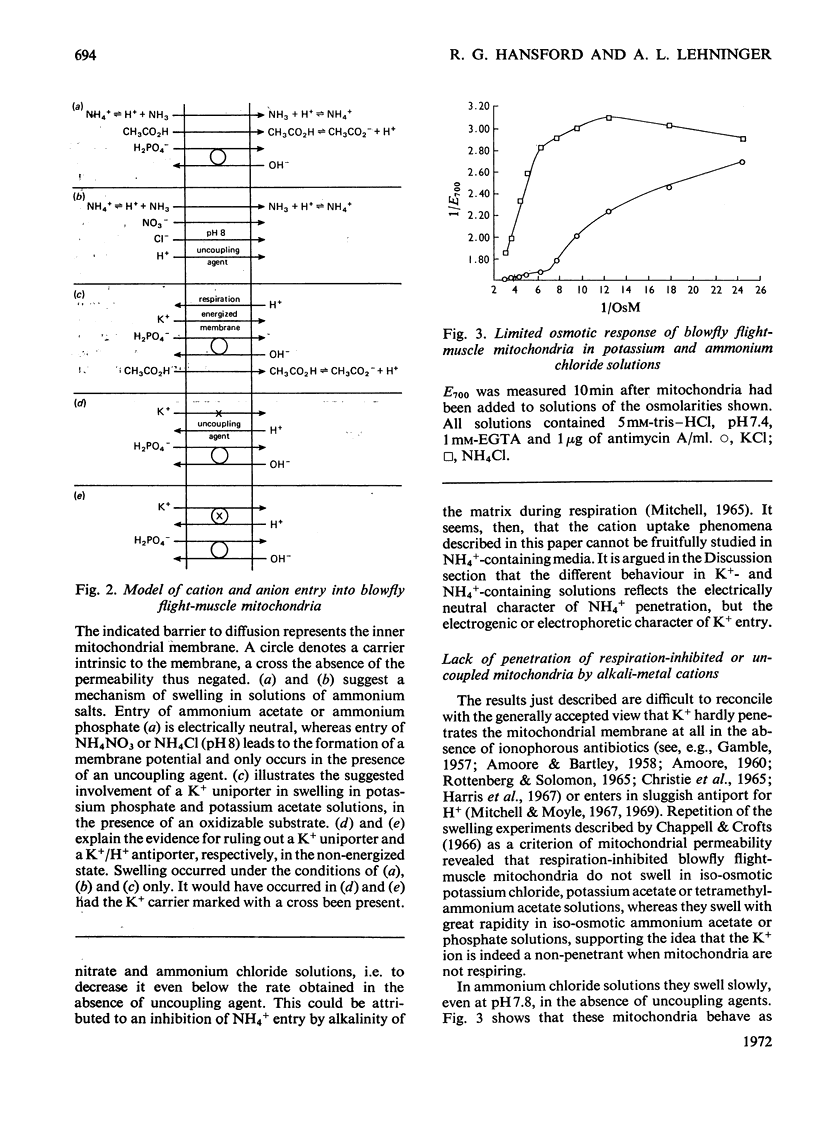
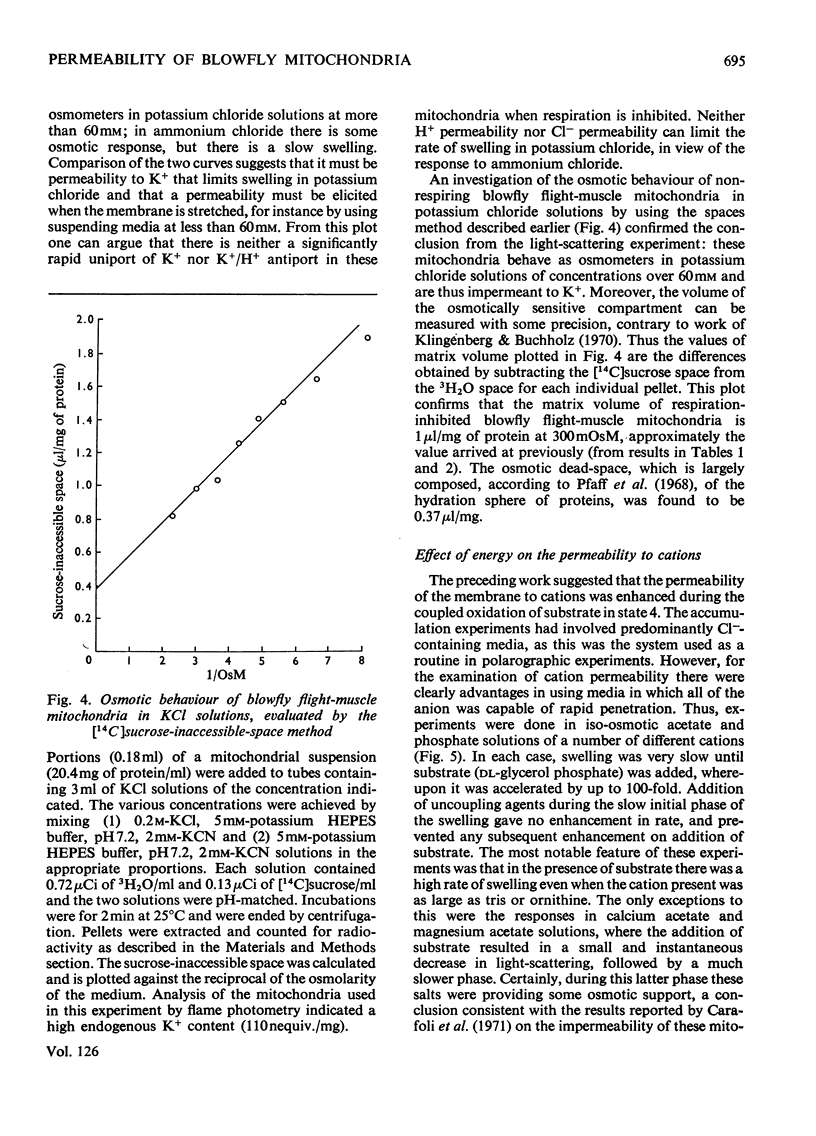
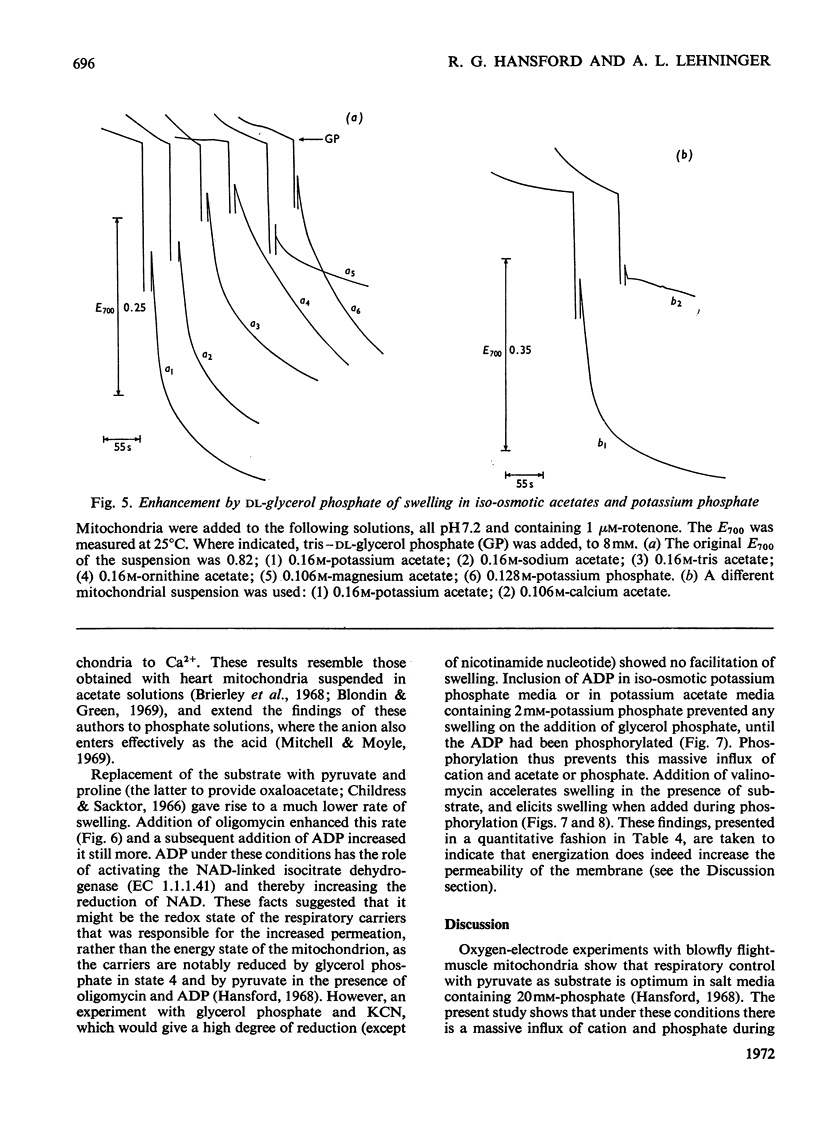
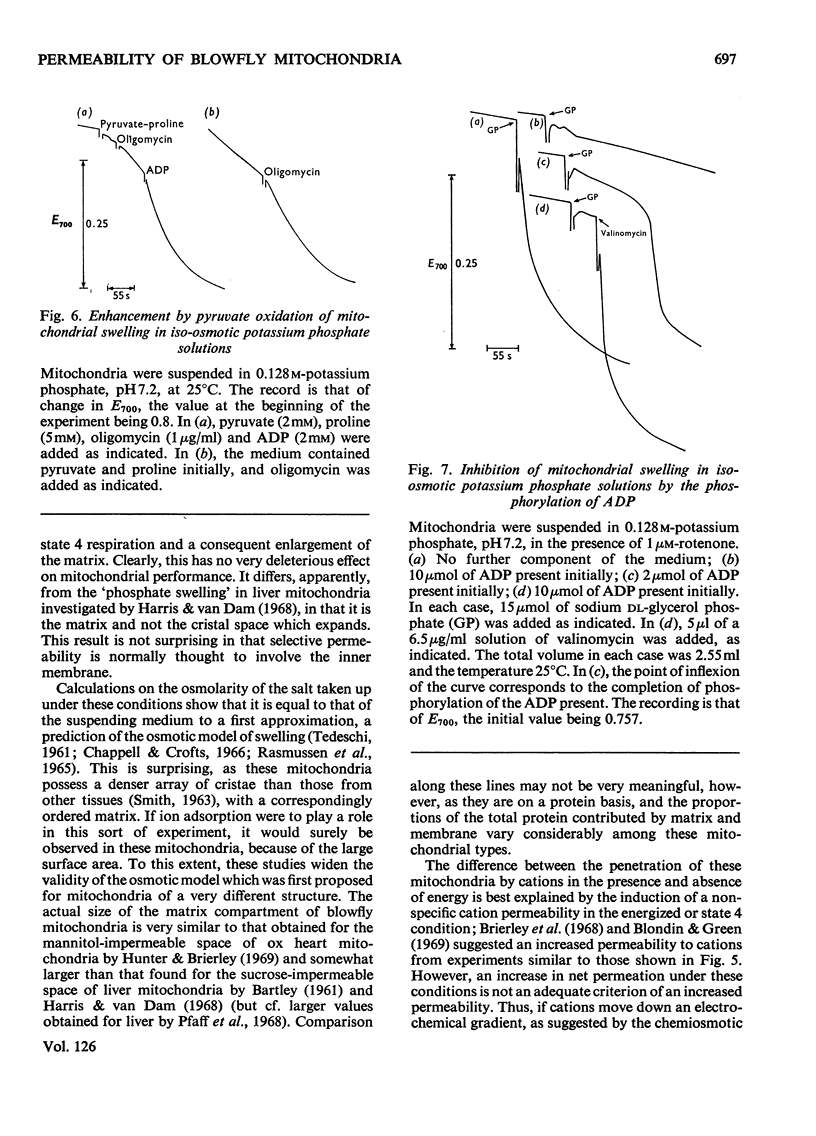
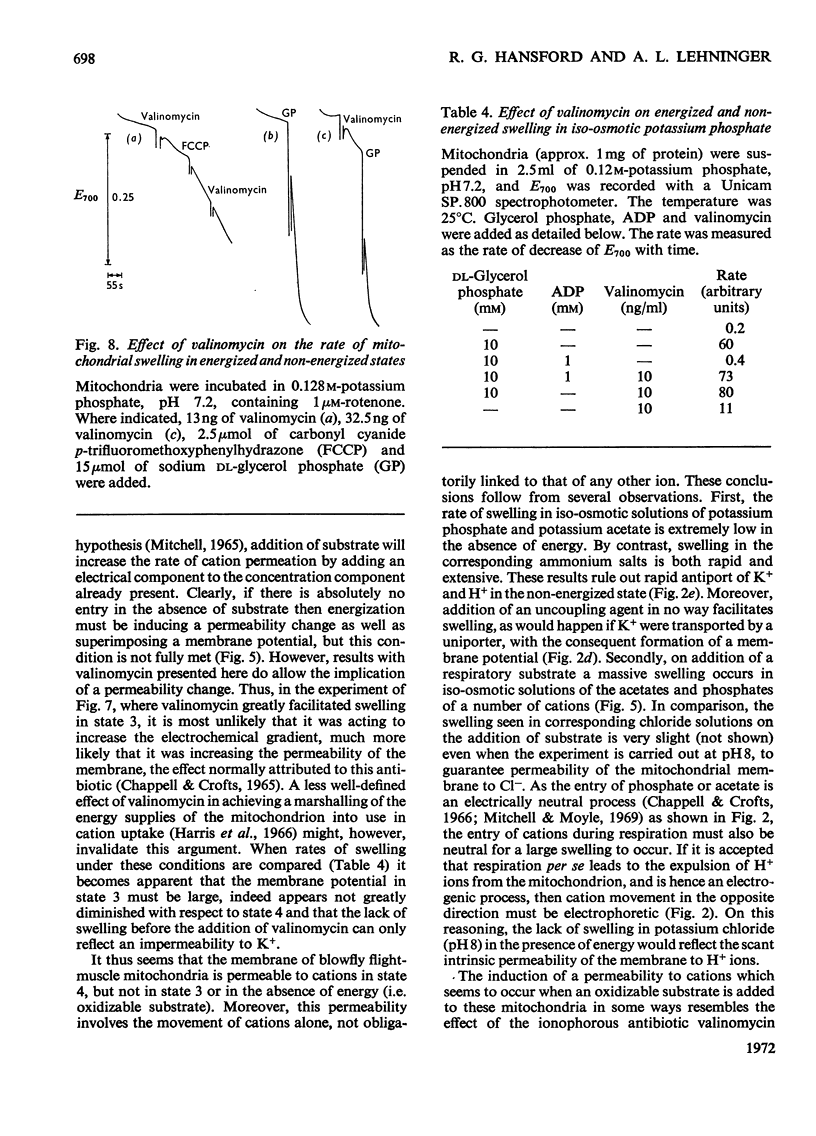
1. Blowfly flight-muscle mitochondria respiring in the absence of phosphate acceptor (i.e. in state 4) take up greater amounts of K+, Na+, choline, phosphate and Cl− (but less NH4+) than non-respiring control mitochondria. 2. Uptake of cations is accompanied by an increase in the volume of the mitochondrial matrix, determined with the use of [14C]-sucrose and 3H2O. The osmolarity of the salt solution taken up was approximately that of the suspending medium. 3. The [14C]sucrose-inaccessible space decreased with increasing osmolarity of potassium chloride in the suspending medium, confirming that the blowfly mitochondrion behaves as an osmometer. 4. Light-scattering studies showed that both respiratory substrate and a permeant anion such as phosphate or acetate are required for rapid and massive entry of K+, which occurs in an electrophoretic process rather than in exchange for H+. The increase in permeability to K+ and other cations is probably the result of a large increase in the exposed area of inner membrane surface in these mitochondria, with no intrinsic increase in the permeability per unit area. 5. No increase in permeability to K+ and other cations occurs during phosphorylation of ADP in state 3 respiration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E., BARTLEY W. The permeability of isolated rat-liver mitochondria to sucrose, sodium chloride and potassium chloride at 0 degrees. Biochem J. 1958 Jun;69(2):223–236. doi: 10.1042/bj0690223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMOORE J. E. Exchange of potassium ions across a concentration difference by isolated rat-liver mitochondria. Biochem J. 1960 Sep;76:438–444. doi: 10.1042/bj0760438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. IV. Reversible swelling changes linked to transport of monovalent cations stimulated by valinomycin. Biochim Biophys Acta. 1966 Mar 7;113(3):445–456. doi: 10.1016/s0926-6593(66)80003-6. [DOI] [PubMed] [Google Scholar]
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. VI. Metabolism-independent swelling coupled to ion movement. Biochim Biophys Acta. 1967 May 9;131(3):468–478. doi: 10.1016/0005-2728(67)90006-0. [DOI] [PubMed] [Google Scholar]
- BARTLEY W., DAVIES R. E. Active transport of ions by sub-cellular particles. Biochem J. 1954 May;57(1):37–49. doi: 10.1042/bj0570037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLEY W. Solute movemetns during volume changes in rat-liver mitochondria. Biochem J. 1961 Jul;80:46–57. doi: 10.1042/bj0800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin G. A., Green D. E. Mechanism of mitochondrial swelling. 3. Two forms of energized swelling. Arch Biochem Biophys. 1969 Jul;132(2):509–523. doi: 10.1016/0003-9861(69)90395-6. [DOI] [PubMed] [Google Scholar]
- Brierley G. P. Energy-linked alteration of the permeability of heart mitochondria to chloride and other anions. Biochemistry. 1970 Feb 17;9(4):697–707. doi: 10.1021/bi00806a001. [DOI] [PubMed] [Google Scholar]
- Brierley G. P., Settlemire C. T., Knight V. A. Ion transport by heart mitochondria. XI. The spontaneous and induced permeability of heart mitochondria to cations. Arch Biochem Biophys. 1968 Jul;126(1):276–288. doi: 10.1016/0003-9861(68)90584-5. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE G. S., AHMED K., MCLEAN A. E., JUDAH D. ACTIVE TRANSPORT OF POTASSIUM BY MITOCHONDRIA. I. EXCHANGE OF K+ AND H+. Biochim Biophys Acta. 1965 Mar 29;94:432–440. doi: 10.1016/0926-6585(65)90051-8. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Hansford R. G., Sackton B., Lehninger A. L. Interaction of Ca2+ with blowfly flight muscle mitochondria. J Biol Chem. 1971 Feb 25;246(4):964–972. [PubMed] [Google Scholar]
- Childress C. C., Sacktor B. Pyruvate oxidation and the permeability of mitochondria from blowfly flight muscle. Science. 1966 Oct 14;154(3746):268–270. doi: 10.1126/science.154.3746.268. [DOI] [PubMed] [Google Scholar]
- Cockrell R. S., Harris E. J., Pressman B. C. Energetics of potassium transport in mitochondria induced by valinomycin. Biochemistry. 1966 Jul;5(7):2326–2335. doi: 10.1021/bi00871a022. [DOI] [PubMed] [Google Scholar]
- GAMBLE J. L., Jr Potassium binding and oxidative phosphorylation in mitochondria and mitochondrial fragments. J Biol Chem. 1957 Oct;228(2):955–971. [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Catlin G., Pressman B. C. Effect of transport-inducing antibiotics and other agents on potassium flux in mitochondria. Biochemistry. 1967 May;6(5):1360–1369. doi: 10.1021/bi00857a019. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Cockrell R., Pressman B. C. Induced and spontaneous movements of potassium ions into mitochondria. Biochem J. 1966 Apr;99(1):200–213. doi: 10.1042/bj0990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G. R., Brierley G. P. Ion transport by heart mitochondria. XIV. The mannitol-impermeable compartment of the mitochondrion and its relation to ion uptake. Biochim Biophys Acta. 1969 May;180(1):68–80. doi: 10.1016/0005-2728(69)90195-9. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Massari S., Azzone G. F. The mechanism of ion translocation in mitochondria. 2. Active transport and proton pump. Eur J Biochem. 1970 Feb;12(2):310–318. doi: 10.1111/j.1432-1033.1970.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Translocations through natural membranes. Adv Enzymol Relat Areas Mol Biol. 1967;29:33–87. doi: 10.1002/9780470122747.ch2. [DOI] [PubMed] [Google Scholar]
- PRICE G. M., LEWIS S. E. Distribution of phosphorus compounds in blowfly thoracic muscle. Biochem J. 1959 Jan;71(1):176–185. doi: 10.1042/bj0710176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M., Ritt E., Vogell W. Korrelation des unspezifisch permeablen mitochondrialen Raumes mit dem "Intermembran-Raum". Eur J Biochem. 1968 Jul;5(2):222–232. doi: 10.1111/j.1432-1033.1968.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- ROTTENBERG H., SOLOMON A. K. ENERGY LINKED K UPTAKE IN MITOCHONDRIA. Biochem Biophys Res Commun. 1965 Jun 18;20:85–92. [PubMed] [Google Scholar]
- Rasmussen H., Chance B., Ogata E. A mechanism for the reactions of calcium with mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1069–1076. doi: 10.1073/pnas.53.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- SPECTOR W. G. Electrolyte flux in isolated mitochondria. Proc R Soc Lond B Biol Sci. 1953 Apr 17;141(903):268–279. doi: 10.1098/rspb.1953.0041. [DOI] [PubMed] [Google Scholar]
- TEDESCHI H. Osmotic reversal of mitochondrial swelling. Biochim Biophys Acta. 1961 Jan 1;46:159–169. doi: 10.1016/0006-3002(61)90659-x. [DOI] [PubMed] [Google Scholar]



