Summary
This study introduces a novel method for rapidly and efficiently inducing human spinal lower motor neurons (LMNs) from induced pluripotent stem cells (iPSCs) to eventually elucidate the pathomechanisms of amyotrophic lateral sclerosis (ALS) and facilitate drug screening. Previous methods were limited by low induction efficiency, poor LMN purity, or labor-intensive induction and evaluation processes. Our protocol overcomes these challenges, achieving around 80% induction efficiency within just two weeks by combining a small molecule-based approach with transcription factor transduction. Moreover, to exclude non-LMN cells from the analysis, we utilized time-lapse microscopy and machine learning to analyze the morphology and viability of iPSC-derived LMNs on a single-cell basis, establishing an effective pathophysiological evaluation system. This rapid, efficient, and streamlined protocol, along with our single-cell-based evaluation method, enables large-scale analysis and drug screening using iPSC-derived motor neurons.
Keywords: iPSC, motor neuron, single-cell analysis, ALS, disease modeling, machine learning, image analysis
Highlights
-
•
Developed a protocol to efficiently induce human LMNs from iPSCs for ALS studies
-
•
Achieved ∼80% LMN induction in two weeks
-
•
Improved efficiency by combining small molecules and transcription factors
-
•
Developed a system for analysis and drug screening using single-cell techniques
In this article, Okano, Morimoto, and colleagues show that a novel protocol enables rapid and efficient induction of human spinal lower motor neurons (LMNs) from iPSCs, achieving 80% efficiency in two weeks. This efficient induction method, along with their single-cell-based evaluation system, enables robust phenotype assessment, facilitating large-scale iPSC-based ALS research and drug screening.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by late-onset, progressive, and fatal motor neuron (MN) degeneration, leading to muscle weakness, respiratory failure, and ultimately death. Despite intensive research efforts, the underlying pathomechanisms of ALS remain poorly understood, creating a high demand for fast methods to evaluate therapy effectiveness. Induced pluripotent stem cells (iPSCs) have emerged as a powerful tool for drug screening and understanding the mechanisms underlying ALS pathology (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Sances et al., 2016; Goto et al., 2017; Fujimori et al., 2018; Shin et al., 2018; Ito et al., 2023; Okano and Morimoto, 2022; Okano et al., 2020). However, iPSC-based research still faces significant challenges, including low induction efficiency, poor MN purity, and labor-intensive induction and evaluation procedures (Lamas and Roybon, 2021; Castillo Bautista and Sterneckert, 2023).
These problems have constrained most iPSC-based ALS disease model studies to cells with clear genetic abnormalities and small sample sizes, limiting their applicability to sporadic cases of ALS. Sporadic ALS, which comprises the majority of ALS cases, lacks obvious genetic abnormalities, exhibits high heterogeneity in disease course, and occurs in patients with various backgrounds. Accurate analysis would thus seem to require a large number of iPSCs derived from many sporadic ALS patients. An efficient neural induction method with clear endpoints is essential for utilizing a large iPSC library.
In this study, we developed a novel protocol for the rapid and efficient induction of human spinal lower motor neurons (LMNs) from iPSCs, targeting the spinal cord, a major locus of ALS pathogenesis. Our protocol achieved around 80% induction efficiency within just two weeks, a significant improvement over conventional methods (Sances et al., 2016; Goto et al., 2017). Time-lapse microscopy and machine learning allowed us to analyze the morphology and viability of iPSC-derived neurons at the single-cell level, establishing an effective pathophysiological evaluation system for neurodegenerative diseases. This approach has the potential to serve as a key tool for large-scale analysis and drug screening using iPSCs derived from ALS patients, facilitating the development of ALS therapies.
Results
Rapid and efficient induction of human spinal LMNs
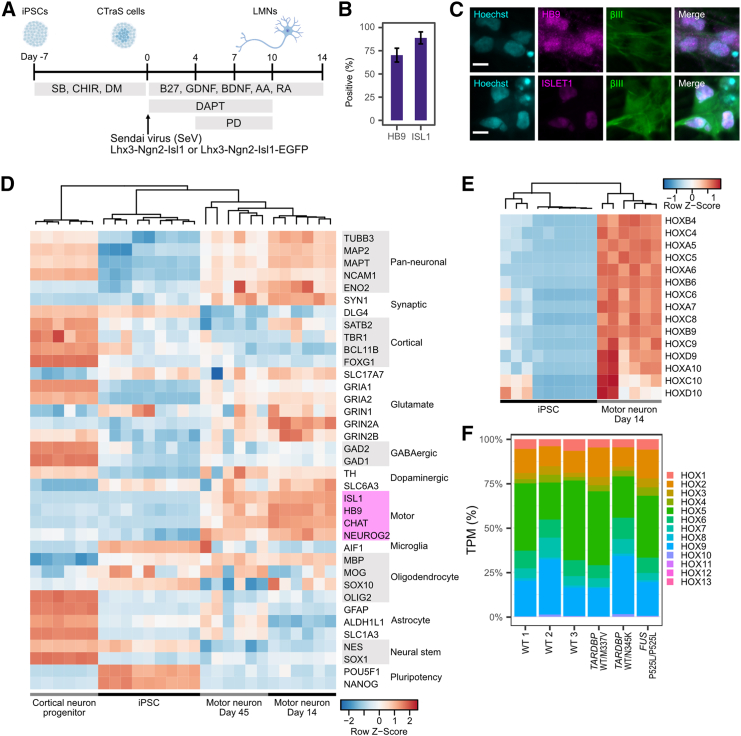
To achieve rapid and efficient induction of human spinal LMNs, we combined a small molecule-based approach with the transduction of transcription factors (Figure 1A). Briefly, iPSCs were treated with 3 μM SB431542 (SB), a transforming growth factor β (TGF-β) signaling inhibitor; 3 μM CHIR9901 (CHIR), a GSK-3β (glycogen synthase kinase 3) inhibitor; and 3 μM dorsomorphin (DM), a BMP signaling inhibitor, for 7 days to induce an embryoid body (EB)-like state (Fujimori et al., 2017). Subsequently, Sendai viruses (SeV) carrying mouse transcription factors Lhx3, Ngn2, and Isl1 (SeV-Lhx3-Ngn2-Isl1), which play critical roles in LMN development, were transfected at a multiplicity of infection (MOI) of 5 (Goto et al., 2017).
Figure 1.
High efficiency and accuracy in differentiating spinal lower motor neurons
(A) Schematic illustration of the differentiation protocol. Sendai viruses were applied at MOI 5.
(B) Differentiation efficiency at day 7 based on immunocytochemistry of HB9 and ISLET. Error bar is the standard deviation. n = 8 biological replicates (different cell lines).
(C) Representative immunocytochemistry image of induced LMNs stained for HB9 (magenta), ISLET1 (magenta), and TUBB3 (green), and counterstained with Hoechst (cyan). Day 7 is shown. Scale bar, 10 μm.
(D) Expression profile of major marker genes in accordance with RNA-seq analysis. Data of cortical neuron progenitors and iPSCs were downloaded from the Sequence Read Archive (SRA) dataset. BioProject IDs are PRJNA660028, PRJNA801842, and PRJNA803470. Cell lines used for MN day 14 and 45 are three WT lines (201B7, WD39, and 414C2) and three ALS lines (A3411 (TARDBPWT/M337V), SM4-4-5 (TARDBPWT/N345K), and FUS-008-1-E6 (FUSP525L/P525L)). Marker genes for motor neuron are highlighted in magenta.
(E) Expression profile of major HOX regional marker genes for spinal cord in accordance with RNA-seq analysis.
(F) Hox gene expression pattern was calculated based on TPM. TPM expression of each group of Hox genes was summed and divided by total Hox gene expression.
Induction efficiency was determined based on the expression of HB9 and ISLET1 (Stifani et al., 2014). Cells were fixed and stained on day 7 of LMN differentiation; 71.1% ± 8.1% of cells were positive for HB9 and, 82.2% ± 7.8% were positive for ISLET1 (Figures 1B and 1C). High induction efficiency of around 80% was also confirmed by fluorescence-activated cell sorting (FACS) analysis of Hb9 promoter::mGreenLantern lentivirus-infected MNs (Figure S1). RNA sequencing (RNA-seq) analysis showed that the neurons had the characteristic expression profile of LMNs, in that high expression of pan-neuronal and LMN markers accompanied low expression of cortical neuron, microglia, oligodendrocyte, astrocyte, neural stem cell, and pluripotency markers (Figure 1D). Notably, we observed higher expression of CHAT (Choline Acetylase), which is required for the cholinergic activity of LMNs. (Wetts and Vaughn, 1994; Stifani et al., 2014). These trends were maintained until day 45 of differentiation. However, there was a decline in neuronal marker expression and an overall increase in glial marker expression at day 45 compared with day 14. We examined the induction efficiency at day 14 and 45 and discovered that the percentage of ISL1-positive cells declined from day 14 to day 45 (Figure S2). This decline of ISL1-positive cells indicates an increased proportion of unwanted cell types and thus explains the decreased neuronal marker expression in bulk mRNA-seq at day 45 (pan-neuronal and motor rows in Figure 1D). We also observed higher expression of AIF1 (a microglial marker) in some cell lines, consistent with a study by Lituma et al., which suggested that AIF1 expression is important for the development of both microglia and excitatory neurons (Lituma et al., 2021). These expression profiles were confirmed by qPCR analysis from day 5 to day 30 (Figure S3). Regarding mRNA expression, endogenous expression of LHX3, NGN2, and ISL1 was minimal compared to the exogenous expression induced by SeV, which continued at least until day 45 (Figure S4). It is highly possible that endogenous gene expression here is suppressed by the abundant exogenous expression.
We also successfully derived LMNs using SeV carrying human transcription factors LHX3, NGN2, and ISL1 (SeV-LHX3-NGN2-ISL1 and SeV-LHX3-NGN2-ISL1-mEmerald) (Figure S5). Induction efficiency was comparable to that of mouse SeV-Lhx3-Ngn2-Isl1, reaching around 80% at day 14 (Figures S5A and S5B). Nonetheless we used mouse TFs (transcription factors) throughout the paper as the use of mouse TFs was well characterized in the previous report by Goto et al. (2017).
Next, we examined the regional character of the induced MNs. Regional association of MNs in the spinal cord is determined by the expression of HOX genes (Sagner and Briscoe, 2019). All HOX genes that determine regions in the spinal cord (HOX4-10) were upregulated compared to iPSCs (Figure 1E). The regional composition of induced LMNs, based on Hox gene expression patterns, was broad, ranging from the cervical to the thoracic lumbar spinal cord, with HOX4-10 genes being dominant compared to the lumbar and sacral spinal cord genes (HOX10-13) (Figure 1F).
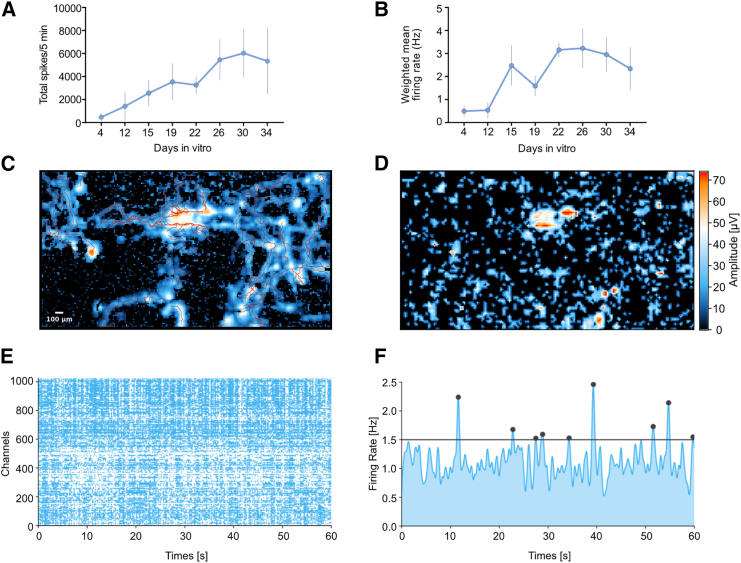
To further characterize the induced LMNs, their spontaneous functional activity was tested. We plated them onto two independent MEA systems: Maestro and MaxOne. In the Maestro system, each well contains 16 electrodes, while, in the MaxOne system, each plate contains 26,400 electrodes, providing better coverage for measuring functional activity. After SeV induction, LMNs were cultured for 2 weeks before being plated into MEA. Functional measurements were taken over a period of 1 month for the Maestro system and at a single time point for the MaxOne system. We tracked the development of spontaneous activity and observed a progressive increase in the number of spikes and the weighted mean firing rate in the Maestro MEA (multi electrode array) system (Figures 2A and 2B). Using the axon tracking function, we observed the formation of long axons across the neuronal network, where the mean length of the longest axon was approximately 864 μm and the furthest distance from the initiation site was 920 μm (Figure 2C and Table S1). Additionally, the network activity map showed an active LMN culture, as demonstrated by the raster plot and mean firing rate activity (Figures 2D−2F, and Table S2). We conclude that these results indicate the LMNs are in a mature functional state, as demonstrated by robust neuronal firing activity—evidenced by mean firing rates and network burst activity—and supported by axon tracking data showing a conduction velocity exceeding 0.5 m/s (Radivojevic and Rostedt Punga, 2023; Schenke et al., 2021; Xu et al., 2023).
Figure 2.
LMN showed spontaneous functional activity
(A) Total number of spikes that correspond to local field potential activity was measured within 5 min of MEA recording. Plot shows mean ± SEM from 3 independent wells.
(B) The weighted mean firing rates were computed as the average firing rates corrected for the number of active electrodes. Plot shows mean ± SEM from 3 independent wells.
(C) Axon tracking analysis by using the MaxOne MEA system, correspond to Table S2.
(D) Network activity map measured by MaxOne MEA, showing strong action potential activity.
(E) Representative raster plot from one of the active neuron sites; the plot above each raster plot shows each spike detected for each electrode over a 1 min recording period.
(F) Mean firing rate activity was measured over a 1 min recording period.
LMNs derived from ALS-iPSCs show pronounced protein aggregation
To determine whether LMNs with ALS mutations exhibit pathological phenotypes, we used four ALS-iPSC lines carrying genetic mutations in either TDP-43 (TARDBP, TAR DNA binding protein 43) or FUS (fused in sarcoma). Aberrant cytoplasmic accumulation and aggregation of TDP-43 in MNs is a prevalent pathology in ALS patients, both in sporadic and familial cases with TDP-43 mutations (Sagner and Briscoe, 2019; Arai et al., 2006; Sreedharan et al., 2008; Lagier-Tourenne and Cleveland, 2009; Tamaoka et al., 2010). FUS mutations are another well-studied cause of familial ALS, where mutant FUS protein accumulates and aggregates aberrantly in the cytoplasm (Vance et al., 2009; Kwiatkowski et al., 2009).
We performed immunocytochemistry (ICC) on LMNs derived from the following cell lines to determine whether ALS LMNs retain these pathological phenotypes such as TDP-43 and FUS aggregation in the cytoplasm. The ALS-iPSC lines used were TARDBPWT/M337V (A3411, derived from an ALS patient), TARDBPWT/N345K (SM4-4-5, derived from an ALS patient), FUSP525L/P525L (FUS-008-1-E6, isogenic mutated cell line of WT 1; wild type), and FUSWT/P525L (FUS-008-1-G2, isogenic mutated cell line of WT 1) (Table S3). These lines were induced to differentiate into LMNs using the protocol described earlier. On day 7 of differentiation, cells were fixed and immunostained and images were analyzed using machine learning for single-cell level ICC image analysis to exclude dead and HB9 or ISL1-negative cells (Figure 3A).
Figure 3.
Spinal lower motor neurons derived from ALS-iPSCs exhibit distinct protein aggregation
(A) Representative images of the granule recognition strategy. Each cell was recognized based on Hoechst staining. The soma was defined by the collar function, and granules were recognized by the robust puncta function of IN Carta. The last image shows segmented cells with nuclei (cyan), soma (gray), and granules (pink). Misrecognized dead cells or HB9-negative cells, such as a–c in the image, were removed from analysis by defining them using Phenoglyphs of IN Carta. In the figure a–c are dead cells with fragmented small nuclei and d–f are live positive cells. Scale bar, 10 μm.
(B) Representative images of TDP-43 and FUS granule recognition in each cell line. Scale bar, 10 μm.
(C) Mean cytosolic granule area per cell. Results were from three to four independent experiments. Two-tailed Dunnett’s test was performed. ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001; ns, non-significant.
As expected, TARDBPWT/M337V LMNs had more aggregated TDP-43 in their cytoplasm than control WT cell lines (Figures 3B and 3C). For the ALS patient with the TARDBPN345K mutation, it has been reported that, in the autopsy, TDP-43 aggregation or mis-localization was not observed in LMNs, but rather in astrocytes (Takeda et al., 2019). Our findings for TARDBPWT/N345K were consistent with the patient’s symptoms, showing a lack of cytosolic granules in LMNs. On the other hand, although ALS patients with FUS mutations typically do not show cytosolic TDP-43 aggregation in MNs, FUSP525L/P525L LMNs showed a significant increase in TDP-43 aggregation in the cytosol (Lagier-Tourenne and Cleveland, 2009).
We also analyzed TDP-43 mis-localization using the cytoplasm-to-nucleus mean intensity ratio for each cell (Figure S6). While ALS patients with TARDBP mutations (excluding TARDBPN345K) often exhibit TDP-43 depletion from the nucleus and accumulation in the cytosol, our analysis showed little difference between WT and ALS LMNs in terms of fluorescence intensity (Figure S6 top right panel).
We also examined FUS aggregation and accumulation in the cytoplasm. As expected, FUS/P525L/P525L LMNs showed significantly more FUS aggregation and accumulation in the cytoplasm (Figures 3C and S6 bottom right panel). Although FUSWT/P525L LMNs did not show a significant increase of FUS granules in the cytoplasm, we detected mis-localization based on the cytoplasm-to-nucleus mean intensity ratio. The lack of some disease phenotypes of FUSWT/P525L may be explained by the fact that it is a heterozygous FUS mutation.
The observed aggregation and mis-localization of TDP-43 and FUS in the ALS cell lines indicate that our LMN induction method and single-cell-based image analysis are suitable for disease research.
Bulk analysis shows reduced neurite outgrowth in LMNs derived from ALS-iPSCs compared to WT controls
Next, we investigated the vulnerability of LMNs derived from ALS-iPSCs, as MN degeneration is a key symptom in ALS patients. To assess the phenotype of induced MNs from ALS-iPSCs, we examined neurite growth (Fujimori et al., 2018; Morimoto et al., 2023). Cell images were acquired every 12 h from day 3 to day 13 to evaluate neurite length and cell number. We used a Sendai virus that transduced EGFP along with transcription factors Lhx3, Ngn2, and Isl1 to allow for clearer detection of neurites (Figures 1A and 4A).
Figure 4.
Spinal lower motor neurons derived from ALS-iPSCs show reduced outgrowth of neurites
(A) Representative image of neurite (green) and soma (randomly assigned color) recognition based on EGFP florescence on days 7 and 13. Scale bar, 160 μm.
(B) Total neurite length of each well was normalized by dividing by the corresponding neurite length at day 3. n = 3–5 independent experiments. Normalized maximum neurite length was compared and tested using Dunnett’s test.
(C) Total cell number of each well was normalized by dividing by the cell number on day 3. n = 3–5 independent experiments. Normalized minimum cell number was compared and tested using Dunnett’s test. For (B) and (C), error bars represent standard error. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, non-significant.
Overall, our LMNs retained the expected phenotypes based on their genotype. We compared the normalized maximum neurite length and the normalized minimum cell numbers (Figure 4B right and 4C right). The maximum neurite length was significantly shorter for ALS cell lines, except for the FUSWT/P525L cell line. FUSWT/P525L cells, with a heterozygous FUS mutation, had a maximum neurite length comparable to WT cell lines. We also replicated the reduced outgrowth of ALS LMN neurites using human transcription factors SeV and SeV-LHX3-NGN2-ISL1-mEmerald with WT 1 and TARDBPWT/M337V (Figures S5C and S5D).
Although TARDBPWT/M337V, TARDBPWT/N345K, and FUSP525L/P525L had shorter neurites, there were no significant differences in the normalized minimum cell number, except for FUSP525L/P525L (Figure 4C). However, ALS cell lines TARDBPWT/M337V and FUSP525L/P525L died faster than WT cell lines according to microscopic observation. Additionally, when we evaluated apoptosis with immunostaining of cleaved caspase-3 (CC3), we found that TARDBPWT/M337V and FUSP525L/P525L had a greater granule area and more CC3-positive cells compared to WT lines, indicating stronger apoptotic signals (Figure S7).
We observed an increase in cellular debris over time, which was hard to distinguish from live cell bodies by the analysis software. This debris was misrecognized as cells by automated image analysis, making accurate cell counting difficult. We hypothesized that analyzing only cells that could be tracked from the start to the end of the experiment or until cell death would provide a clearer survival analysis. Therefore, to clearly evaluate cell survival probability, we developed a method for single-cell resolution analysis.
Cell vulnerability more accurately evaluated by single-cell tracking and survival analysis
For accurate single-cell tracking, it is essential to detect target cells precisely. To avoid overcrowding of EGFP-positive cells, we mixed SeV-L-N-I and SeV-L-N-I-EGFP cells at a 10:1 ratio (Figures 5A and 5B). The starting point (0 h) of survival analysis was set at 4 days post-Sendai virus transfection. To minimize the possibility of cells moving out of the field of view, we acquired 5 × 5 tiling images.
Figure 5.
Single-cell tracking and survival analysis revealed that ALS-iPSC-induced LMN dies faster than WT controls
(A) Experimental scheme.
(B) Representative image of cell tracking. The white arrowhead indicates the tracked cell. (Top) EGFP images are overlaid on phase contrast images. (Down) EGFP images.
(C) Survival curves were compared between ALS cell lines and WT control cell lines (201B7 and WD39). p values were calculated by the log rank test corrected with the Benjamini-Hochberg procedure.
As expected, TARDBPWT/M337V and FUSP525L/P525L LMNs were significantly more likely to die compared to healthy LMNs, whereas the survival curve of TARDBPWT/N345K was not different from that of healthy cell lines (Figure 5C). For FUSWT/P525L, although we did not observe significant differences in bulk analysis earlier, we observed a significant difference in the survival curve. We further tested 4 additional ALS cell lines to confirm our results. Using this method, we successfully confirmed the vulnerability of cells carrying other ALS mutations and cells derived from sporadic ALS patients (Figure 5C).
Discussion
We have demonstrated the high efficiency of a novel spinal LMN induction protocol. The single-cell-based evaluation method showed that induced MNs with ALS mutations presented phenotypes consistent with major characteristics of the disease as established in previous reports, such as cytoplasmic TDP-43/FUS aggregation and neurite vulnerability. Earlier studies using Sendai virus to derive MNs reported an induction efficiency of about 30% (Goto et al., 2017). By contrast, our method improved efficiency to 70%–80% by transforming iPSCs into a chemically transitional EB-like state (CTraS) with three small molecules—SB, DM, and CHIR99021—before SeV treatment. CTraS accelerates differentiation by inhibiting TGF-β/BMP and activating Wnt (wingless type MMTV integration site family) signaling (Fujimori et al., 2017). This paper demonstrated that these molecules work well as a pretreatment for transcription factors and virus-based transcription factor induction. Although other protocols achieve higher purity of MNs, the speed and efficiency with which it produces LMNs (70%–80%) is a significant advantage of our protocol (Table 1) (Du et al., 2015; Sances et al., 2016; Sepehrimanesh and Ding, 2020).
Table 1.
Comparison of MN induction methods
| Reference | Efficiency (%) | Total days | Differentiation approach | Key molecules | Disease recapitulation |
|---|---|---|---|---|---|
| Du et al., 2015 | 95 (/DAPI)) | 28 | small molecule | N2, B27, CHIR, DMH1, SB, RA, PMN, VPA, BDNF, CNTF, IGF-1, compound E | SOD, neurite fragmentation |
| Goto et al. (2017) | 30 (/DAPI) | 21 | Sendai virus | SB, dorsomorphin, SeV-TFs, RA, SAG, N2, B27, BDNF, GDNF, NT-3 | SOD, TDP-43 aggregation |
| Sepehrimanesh and Ding (2020) | >90 (/TUBB+) | 39 | lentivirus | Lentivirus-TFs, β-ME, N2, NEAA, heparin, bFGF, EGF, B27, forskolin, BDNF, GDNF, NT-3 | not mentioned |
| Present paper | 80 (/DAPI) | 21 | Sendai virus | CHIR, SB, dorsomorphin, SeV-TFs, B27, BDNF, GDNF, RA, AA, DAPT, PD | FUS, TDP-43 aggregation, neurite length, survival |
There were interesting discrepancies in the neurite analysis and survival analysis of TARDBPWT/N345K and FUSWT/P525L (Figures 4B and 5C). TARDBPWT/N345K did not have a lower survival probability despite showing shorter neurites, while FUSWT/P525L did not show shorter neurites despite having a lower survival probability. These discrepancies may be due to a limitation in the protocol or could reflect differences in the molecular pathways by which these mutations affect LMNs. For example, the WT/N345K mutation of TARDBP may work “backwards” in that it first affects neurites, and the WT/P525L mutation of FUS may affect “forwards” in that it is lethal for the cell but has a delayed effect on the neurites. Investigating these differences may reveal more detailed molecular mechanisms underlying ALS pathology.
Our rapid differentiation and scoring methods have potential to accelerate drug screening (Okano and Morimoto, 2022; Bonaventura et al., 2021) and facilitate reverse translational research by comparing the therapeutic effects of candidate drugs in both in vitro and in vivo models (Morimoto et al., 2023). Over the past decade, several drugs have entered clinical trials based on preclinical tests or screenings using MNs derived from iPSCs or embryonic stem cells (ESCs) (Table 2). However, these screenings predominantly rely on a limited number of iPSC lines with genetic mutations, despite the fact that sporadic ALS cases account for approximately 90% of the total. This is partly because screenings using genetically mutated cell lines can produce clearer results with fewer lines. In contrast, drug screening for sporadic ALS requires a larger and more diverse set of cell lines due to the heterogeneous nature of the disease, which makes achieving clear results more challenging with only a few lines. Our rapid differentiation and scoring methods could broaden the scope of drug screening by including a wider range of sporadic ALS cell lines.
Table 2.
Drug developments based on induced MNs
| Reference | Efficiency (%) Isl1 or HB9 |
Total days | Differentiation approach | Rescued phenotype | Number of tested drugs | Number of tested ALS patient-derived cell line | Features of tested cell lines | Drug |
|---|---|---|---|---|---|---|---|---|
| Donnelly et al. (2013) | 30–40 | 40–45 | small molecule | C9orf72 RNA level, nuclear GGGGCC RNA foci, Glutamate exposure susceptibility | 5 (antisense oligo) | 4 | C9orf72 | antisense oligo |
| Wainger et al. (2014) | N.A. (FACS purified) | 52 | small molecule | hyperexcitability | 1 | 2 | SOD1 | ezogabine (retigabine) |
| Naujock et al. (2016) | 60-67 (/DAPI) | >42 | small molecule | spontaneous activity, ion channel imbalance | 1 | 6 | SOD1, FUS | 4-aminopyridine (4AP) |
| Imamura et al. (2017) | 60 (/DAPI) | 14 | TFs polycistronic vector | misfolded SOD1, survival | 1,416 | 1 | SOD1 | bosutinib |
| Guo et al. (2017) | 80 (/DAPI) | 32–38 | small molecule | FUS mis-localization, hyperexcitability, axonal transport | 1 | 3 | FUS | ACY-738/Tubastatin A |
| Fujimori et al. (2018) | 65 (/DAPI) | 60 | small molecule | FUS and TDP-43 aggregation/mis-localization, neurite length, LDH leakage, CC3 | 1,232 | 4 | TDP-43, FUS | ropinirole |
| Thams et al. (2019) | 40 (/DAPI) | 31 | small molecule | survival (under ER stress) | 1,275 | 1 | SOD1 (edited ESC) | TUDCA |
Furthermore, our methods have the potential to enable personalized drug screening. ALS symptoms progress rapidly, rendering daily physical activities impossible within 1–2 years. Therefore, the rapid establishment of patient-derived iPSCs and drug screening of MNs is essential for precision medicine. Although there are many challenges to the practical application of personalized medicine using iPSCs (Shin et al., 2018; Novelli et al., 2022; Palasantzas et al., 2023; Nguyen et al., 2022), our method can shorten the time required for drug screening and contribute to practical applications.
Single-cell-based analysis is a powerful tool in the study of heterogeneous cell populations (Mattiazzi Usaj et al., 2021). In our study, we expanded the application of single-cell tracking (Shin et al., 2018) to cell survival analysis to accurately evaluate the cellular vulnerability of ALS patients using long-term live imaging and single-cell tracking of iPSC-derived spinal LMNs. Further associating the morphological data of each cell with multi-omics expression data would be interesting (Watson et al., 2021; Park et al., 2022). For ALS studies using iPSC-derived LMNs, associating phenotypic data, such as cell morphology and ICC images, with omics data could help analyze differences between the most affected and less affected cells in an isogenic cell line and identify epigenetic differences among cells with identical genomic backgrounds.
In this study, we used a limited number of ALS-iPSC lines with genetic mutations. Although our induced MNs successfully reproduced typical ALS phenotypes and partial correlation between clinical drug response and in vitro assays was observed in another study using this induction method, it is not clear whether the severity of the phenotype correlates with the patient’s clinical progression (Morimoto et al., 2023). It should also be noted that the exogenous expression of transcription factors Lhx3, Ngn2, and Isl1 may have some effect on the induced MNs’ disease phenotype. Future extensive studies analyzing relationships between patients’ pathological phenotypes, including sporadic cases, and the corresponding induced MN phenotypes are necessary. Recent advances in large libraries of iPSCs from ALS patients with clinical records, such as Answer ALS (Baxi et al., 2022) and JaCALs (Nakamura et al., 2023), will help evaluate the usefulness and precision of ALS disease modeling approaches.
Experimental procedures
Research ethics
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Keio University School of Medicine (approval no. 20080016).
Human feeder-free iPSC culture
Feeder-free iPSCs were maintained in StemFit AK02N medium (Ajinomoto, Tokyo, Japan). Cells were dissociated using 0.5 × TripLE Select (Thermo Fisher Scientific, Waltham, MA) and seeded at 0.3–1 × 104 cells/well in six-well plates treated with 2 μL/mL iMatrix-511 (Matrixome, Osaka, Japan) (Laminin 511E8; FUJIFILM Wako Pure Chemical Corp., Tokyo, Japan). Y27632 (10 μM; Nacalai, Kyoto, Japan) was added only for the first day. The medium was changed every other day. The cell lines used in this study are described in Table S3 (Takahashi et al., 2007; Okita et al., 2011; Imaizumi et al., 2012; Egawa et al., 2012; Leventoux et al., 2020).
LMN induction from iPSCs using Sendai viruses
LMNs were induced from iPSCs via a chemically transitional EB-like state (CTraS) (Fujimori et al., 2017) and infection with SeV-L-N-I (ID Pharma, Tsukuba, Japan), SeV-L-N-I-EGFP (ID Pharma) (Goto et al., 2017), or SeV-LHX3-NGN2-ISL1 (Repli-tech Co., Ltd, Tokyo, Japan). Briefly, iPSCs were seeded in StemFit AK02N (Ajinomoto) with 10 μM Y27632 (Nacalai) and 2 μL/mL iMatrix-511 (Matrixome) and cultured for 5 days at 37°C under 5% CO2. The medium was changed the following day to remove Y27632 and then every 2 days thereafter.
To induce an EB-like state, the medium was changed to a chemical induction medium (StemFit AK02N with 3 μM SB [Sigma-Aldrich, St. Louis, MO], 3 μM CHIR [Cayman, Ann Arbor, MI], and 3 μM DM [Santa Cruz, Dallas, TX]) for 7 days (Fujimori et al., 2017). The medium was changed daily during this period.
After 7 days, iPSC colonies were dissociated into single cells using 0.5 × TripLE Select and seeded onto plates coated with 0.0001% poly-L-lysine (PLL) (Sigma-Aldrich), 10 μL/mL Matrigel (Thermo Fisher Scientific), and 6.4 μL/mL iMatrix-511 (Matrixome). PLL was diluted in distilled water, and wells were kept overnight at 37°C. After removing PLL, wells were treated with the Matrigel and iMatrix-511 mixture in phosphate-buffered saline (PBS) and incubated at 37°C for at least 1 h. The mixture was removed just before plating the cells. The same concentration of coating reagents was used for both 96-well and 12-well formats.
The MN medium consisted of KBM neural stem cell medium (KOHJIN BIO, Saitama, Japan) supplemented with 2% B27 ((Thermo Fisher Scientific), penicillin (100 U/mL)-streptomycin (100 μg/mL) (Thermo Fisher Scientific), 200 μM ascorbic acid, 10 ng/mL brain-derived neurotrophic factor (R&D Systems, Minneapolis, MN), 10 ng/mL glial cell-derived neurotrophic factor (Alomone Labs, Jerusalem, Israel), 10 μM DAPT (Sigma-Aldrich), and 10 μM Y27632. SeV was applied at an MOI of 5. Y27632 was removed from the MN medium after 1 day. The medium was changed on days 1, 3, 4, 7, 10, and 13 and every three days thereafter. DAPT was added until day 7. On days 4 and 7, 2 μM PD0332991 (Sigma-Aldrich) was added to remove proliferating cells. If there was no change in the medium components, only half the volume of MN medium was exchanged to avoid damaging LMNs. Cells were incubated at 37°C with 4% O2 and 5% CO2 until day 7 for ICC or day 13 for neurite analysis and single-cell tracking (Santilli et al., 2010).
For ICC, cells were seeded in 96-well plates at 1 × 105 cells/well and infected with SeV-L-N-I. For neurite length analysis, cells were seeded in 12-well plates at 5 × 105 cells/well and infected with SeV-L-N-I-EGFP. For single-cell tracking, SeV-L-N-I and SeV-L-N-I-EGFP were mixed at a 10:1 ratio and cells were infected at an MOI of 5. Cells were seeded at 6 × 105 cells/well in 12-well plates.
RNA-seq and analysis
Total RNA was isolated from LMNs on days 14 and 45 using an RNeasy mini kit (QIAGEN, Hilden, Germany) with DNase I treatment. RNA libraries for RNA-seq were prepared using a Nextera XT DNA library prep kit (Illumina, San Diego, CA) following the manufacturer’s protocols. Raw fastq files were trimmed to remove low-quality bases and adapters using fastp v.0.23.2 (Chen et al., 2018) and processed for further analyses. To generate TPM, salmon v.1.7.0 was used (Patro et al., 2017). The transcript index was created from the reference GRCh38 genome annotation (GENCODE release 39) to quantify gene expression levels. DESeq2 was used to identify differentially expressed genes (Love et al., 2014) with a cutoff of 0.05 for Benjamini-Hochberg-adjusted p values and a cutoff of 0.25 for the log2 fold change ratio.
For the analysis of exogenous expression in Figure S4, reads were mapped to GRCh38 release 84 version of H. sapiens using HISAT2 (version 2.1.0) with relaxed mismatch penalty (scoring option; --mp 1,0) (Kim et al., 2019). Resulting sam files were converted into bam files using samtools version 1.9 (Danecek et al., 2021). The data were investigated using IGV (Ingetrative Genomic Viewer) (Robinson et al., 2011).
Quantitative reverse-transcription PCR
Total RNA was isolated using an RNeasy Mini Kit (QIAGEN) and treated with DNase I. cDNA was prepared using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative reverse-transcription PCR was performed using Premix Ex Taq II (Takara Bio Inc., Shiga, Japan) on a ViiA 7 real-time PCR system (Thermo Fisher Scientific). qPCR primers are listed in Table S4. Relative expression was calculated using the ΔΔCT method. ACTB (actin beta) was used as the internal control to calculate ΔCT, and the control condition to calculate ΔΔCT was day 0 WT 3 (414C2).
High-density microelectrode array recording by MaxOne
MaxWell Biosystems high-density microelectrode arrays (HD-MEAs) (MaxWell Biosystems, Zürich, Swiss) were used to assess the electrophysiological activity of MNs. Prior to HD-MEA experiments, MNs were differentiated for two weeks post-SeV transduction in MN culture medium in regular tissue-culture plates (see method on LMN induction from iPSCs using Sendai viruses). On the day of plating, MNs were dissociated with Accutase and counted using the Countess system (Thermo Fisher Scientific).
MaxOne HD-MEA chips were incubated with 1% Tergazyme solution for 2 h at room temperature and washed with water, followed by submersion in 70% ethanol for sterilization for approximately 1 h. HD-MEA chips were washed with sterile water three times, and then 1 mL of complete culture media consisting of BrainPhys medium with SM1 (STEMCELL Technologies, Vancouver, Canada) was added to prime the chips. HD-MEA chips were incubated for 2 days in a humidified cell culture incubator at 37°C with 5% CO2.
On the day of cell plating, the culture media were removed and chips were washed three times with sterile water prior to coating with 0.1% polyethyleneimine (PEI, Sigma-Aldrich). Chips were placed back in the incubator for 1 h. PEI was then removed, and the chips were washed three times with sterile water and allowed to dry in the biosafety cabinet for 1 h. Laminin was then applied to the chips, which were placed back in the incubator until the cells were ready to be seeded. Laminin coating was removed, and 50 μL of MN suspension was pipetted into the middle section of the chip at a density of 50,000 cells per chip. After 1 h of incubation, 600 μL of MN culture medium was added to each chip. The next day, 50% of the medium in each chip was replaced with fresh culture medium. Media changes were performed every 3 days by removing 50% of the medium and replenishing it with an equal volume of fresh culture medium. HD-MEA recordings were performed on day 14 post-plating. The MaxWell Biosystems recording unit was sterilized with 70% ethanol, placed in the biosafety cabinet, and allowed to dry for 30 min. The recording unit was then transferred to an incubator for at least 2 h for temperature equilibration prior to recording.
To assess neuronal activity across the entire electrode array, the “Activity Scan Assay” module in the MaxLab Live software was used. The network assay module was then used to assess network activity or axonal features. Network electrical activity was recorded by selecting a subset of electrodes with the highest firing rate from the corresponding chip’s activity scan and measured simultaneously for 5 min.
Microelectrode array recording by Maestro
Forty-eight well CytoView MEA plates (Axion Biosystems, Atlanta, GA) were coated with 0.1% PEI at room temperature for 1 h, washed with sterile water, and dried overnight in a sterile environment. Two weeks after SeV transduction, MNs were dissociated with Accutase, resuspended at a count of 50,000 cells in 5 μL of MN media with 10 μg/mL Laminin, and drop-pipetted to the center of the electrode area. After 1 h, modified MN media (substituting KBM neural media with BrainPhys media, STEMCELL Technologies) were added drop-wise to the sides of the well. Spontaneous network activity was then recorded using the Maestro MEA system (Axion Biosystems). Neural signals were sampled at 12.5 kHz and high-pass filtered (200 Hz–3 kHz), and a threshold based on six standard deviations above noise level was set by Axion Integrated Studio Software (AxIS). The burst detector was configured to detect network bursts with a maximum interspike interval of 100 ms, a minimum of 50 spikes, and a minimum of 35% participating electrodes. After recording, the data were analyzed using the Neurostatistics compiler (Axion Biosystems).
ICC
Cells were fixed in 4% paraformaldehyde in PBS for 35 min at room temperature. Following fixation, cells were blocked with 10% goat serum and 0.3% Triton X-100 for 1 h at room temperature. Cells were then incubated overnight at 4°C with primary antibodies described in Table S5. Antibodies were diluted with 5% fetal bovine serum (FBS) in PBS. The cells were rinsed with PBS and incubated with species-specific Alexa Fluor 488-, Alexa Fluor 555-, or Alexa Fluor 647-conjugated secondary antibodies (1:2,000; Invitrogen, Thermo Fisher Scientific) mixed with bisbenzimide H (Hoechst) 33258 (Sigma-Aldrich) to counterstain nuclei. Images were obtained using the IN Cell Analyzer 6000 with a ×60 objective lens (Cytiva, Tokyo, Japan). Cell numbers analyzed for each experiment are listed in Table S6.
Image analysis of ICC
Images acquired by the IN Cell Analyzer 6000 were analyzed using IN Carta (Cytiva) with SINAP and Phenoglyphs modules. Each cell was analyzed based on nuclear staining. The somatic area was defined using the collar function, and granular objects of TDP-43, FUS, and CC3 staining were detected using the robust puncta function. Phenoglyphs was used to cluster recognized cells into various groups. The machine was trained to determine whether each group of cells was alive or dead based on nuclear staining and categorized as positive or negative based on marker gene (HB9/ISLET1) staining. Dead cells were identified based on the morphology of nuclear staining, where the nuclei of dead cells undergoing apoptosis exhibit a compact shape and higher intensity due to fragmented chromatins. After applying the classification to all images, the differentiation efficiency of live cells was calculated. Cells that were alive and positive for marker genes were used for further analysis, including the analysis of mis-localization of TDP-43 and FUS. CC3-positive cells were defined as cells with CC3 granules greater than 20 μm2.
Flow cytometry
The cultured MNs were transfected with lentivirus vector backbone designed to express mGreenLantern (Campbell et al., 2020) from an Hb9 promoter. The cells were dissociated using dissociation buffer including papain and Accutase for 20 min, and clumps or debris were removed with a 40 μm cell strainer.
The number of mGreenLantern-positive cells in 200 μL PBS with 2% FBS was measured using a CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis, IN, USA) with CytExpert software, and 50,000 cell events were recorded per sample. Debris and doublets were filtered out. Gates were set based on appropriate unstained samples.
Automated time-lapse live imaging
Images were acquired using BioStation CT (Nikon) from day 3 to day 13. For neurite length analysis, images were automatically acquired every 12 h with six image points per well using a ×10 objective lens for phase contrast and GFP images. For single-cell tracking, 5 × 5 tiling images were acquired every 6 h for each well.
Cell tracking and image analysis
Image analysis was performed using the CL-Quant software (Nikon). Nikon developed a specialized analysis recipe for our dataset. The recipe is available upon request. To analyze the morphological characteristics of cells in culture, morphological filters, including background subtraction, thresholding, and line segmentation, were designed to identify and quantify various features in the raw image, such as cell body number, neurite length, cell body size, compactness, and intensity. For node detection, a binarization combination of the cell body filter and neurite filter was used to detect overlapping regions between the neurite and cell body, which were then used to identify nodes in raw images. After optimizing the filters to detect the individual cells, tracking was performed using the filter for cell bodies of ≥100 pixels.
The settings used in CL-Quant for this tracking were as follows: minimum object size, −1; maximum object size, 999999; maximum search range, 300; split threshold, 0.90; merge threshold, 0.70; minimum trajectory length, 1; object split, ignore split; object merge, merge with partition; enable lineage, off; enable robust measurement, off; object-to-object overlap, off; remove short trace when merging, off. Images acquired between day 4 and day 10 were used for single-cell tracking analysis.
Statistics
Statistical tests and sample sizes (n) are indicated in the figure legends. Statistical analyses were performed using GraphPad software (v.8.2.1) or R. Significance levels were set as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ns for non-significant.
Resource availability
Lead contact
Requests for further information and resources should be directed to and will be fulfilled by the lead contact, Satoru Morimoto (satoru_morimoto@keio.jp).
Materials availability
Materials are available from the corresponding authors upon request.
Data and code availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNA-seq data have been deposited at the Gene Expression Omnibus (GEO) database with accession number GSE229095 and are publicly available as of the date of publication.
Acknowledgments
The authors would like to thank all the members of the H.O. laboratory for their encouragement and kind support. We would also like to thank Shinya Yamanaka, Keisuke Okita, and Makoto Nakagawa (Kyoto University) for kindly providing 201B7, 1210B2, and 414C2; Haruhisa Inoue (Kyoto University) for kindly providing A3411 and A21412; and Takeda Pharmaceutical Company for kindly providing FUS-008-1-E6 and FUS-008-1-G2 iPSC lines. S.M. reports grants supports from the Japan Society for the Promotion of Science (JSPS) (KAKENHI grant no. JP21H05278, JP22K15736, and 22K07500), the Japan Agency for Medical Research and Development (AMED) (grant no. JP23bm1123046 and JP23kk0305024), The Kanae Foundation for the Promotion of Medical Science, the Uehara Memorial Foundation, the Yukihiko Miyata Memorial Trust for ALS Research, Okasan-Kato Foundation Research Grant, Yoshio Koide Grant, Japan ALS Association, Daiichi Sankyo Foundation of Life Science, UBE Academic Foundation, and the Kato Memorial Trust for Nambyo Research during the conduct of the study. H.O. has grant supports from JSPS (KAKENHI grant no. JP20H00485, JP21F21410, JP21H05273, and JP22KF0333) and AMED (grant no. JP22bm0804003, JP20ek0109395, JP20ek0109329, JP21wm0425009, and JP23bm1423002). The funding sources had no role in the analysis. Disclosure forms provided by the authors are available with the full text of this article at Stem Cell Reports. We thank Mitchell Arico from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
S.M. designed the protocol of cell culture. F.O., S.N., and S.M. performed the experiments, prepared the samples for RNA-seq, performed the immunostaining, and imaged the cells. S.M. and S.S. analyzed the data and wrote the manuscript. S.S. performed the RNA-seq analysis. K.H.U. did the functional assay. A.N., N.S., M.A., and Y. Takeshita established iPSCs. S.M. and H.O. provided a grant for the study. Y. Tomari supervised the research and corrected the manuscript. H.O. corrected the manuscript and oversaw the research program. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
H.O. reports grants and personal fees from K Pharma Inc and SanBio Co. Ltd., outside the submitted work.
Published: December 19, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2024.11.007.
Contributor Information
Satoru Morimoto, Email: satoru_morimoto@keio.jp.
Hideyuki Okano, Email: hidokano@keio.jp.
Supplemental information
References
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Baxi E.G., Thompson T., Li J., Kaye J.A., Lim R.G., Wu J., Ramamoorthy D., Lima L., Vaibhav V., Matlock A., et al. Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat. Neurosci. 2022;25:226–237. doi: 10.1038/s41593-021-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura G., Iemmolo R., Attaguile G.A., La Cognata V., Pistone B.S., Raudino G., D’Agata V., Cantarella G., Barcellona M.L., Cavallaro S. iPSCs: A Preclinical Drug Research Tool for Neurological Disorders. Int. J. Mol. Sci. 2021;22:4596. doi: 10.3390/ijms22094596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B.C., Nabel E.M., Murdock M.H., Lao-Peregrin C., Tsoulfas P., Blackmore M.G., Lee F.S., Liston C., Morishita H., Petsko G.A. mGreenLantern: a bright monomeric fluorescent protein with rapid expression and cell filling properties for neuronal imaging. Proc. Natl. Acad. Sci. USA. 2020;117:30710–30721. doi: 10.1073/pnas.2000942117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Bautista C.M., Sterneckert J. Progress and challenges in directing the differentiation of human iPSCs into spinal motor neurons. Front. Cell Dev. Biol. 2023;10 doi: 10.3389/fcell.2022.1089970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., Mccarthy S.A., Davies R.M., Li H. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C.J., Zhang P.W., Pham J.T., Haeusler A.R., Mistry N.A., Vidensky S., Daley E.L., Poth E.M., Hoover B., Fines D.M., et al. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron. 2013;80:415–428. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.W., Chen H., Liu H., Lu J., Qian K., Huang C.L., Zhong X., Fan F., Zhang S.C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N., Kitaoka S., Tsukita K., Naitoh M., Takahashi K., Yamamoto T., Adachi F., Kondo T., Okita K., Asaka I., et al. Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- Fujimori K., Ishikawa M., Otomo A., Atsuta N., Nakamura R., Akiyama T., Hadano S., Aoki M., Saya H., Sobue G., Okano H. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 2018;24:1579–1589. doi: 10.1038/s41591-018-0140-5. [DOI] [PubMed] [Google Scholar]
- Fujimori K., Matsumoto T., Kisa F., Hattori N., Okano H., Akamatsu W. Escape from Pluripotency via Inhibition of TGF-β/BMP and Activation of Wnt Signaling Accelerates Differentiation and Aging in hPSC Progeny Cells. Stem Cell Rep. 2017;9:1675–1691. doi: 10.1016/j.stemcr.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K., Imamura K., Komatsu K., Mitani K., Aiba K., Nakatsuji N., Inoue M., Kawata A., Yamashita H., Takahashi R., Inoue H. Simple derivation of spinal motor neurons from ESCs/iPSCs using sendai virus vectors. Mol. Ther. Methods Clin. Dev. 2017;4:115–125. doi: 10.1016/j.omtm.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Naujock M., Fumagalli L., Vandoorne T., Baatsen P., Boon R., Ordovás L., Patel A., Welters M., Vanwelden T., et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017;8:861. doi: 10.1038/s41467-017-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Okada Y., Akamatsu W., Koike M., Kuzumaki N., Hayakawa H., Nihira T., Kobayashi T., Ohyama M., Sato S., et al. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain. 2012;5:35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Izumi Y., Watanabe A., Tsukita K., Woltjen K., Yamamoto T., Hotta A., Kondo T., Kitaoka S., Ohta A., et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaf3962. [DOI] [PubMed] [Google Scholar]
- Ito D., Morimoto S., Takahashi S., Okada K., Nakahara J., Okano H. Maiden voyage: induced pluripotent stem cell-based drug screening for amyotrophic lateral sclerosis. Brain. 2023;146:13–19. doi: 10.1093/brain/awac306. [DOI] [PubMed] [Google Scholar]
- Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski T.J., Jr., Bosco D.A., LeClerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C., Cleveland D.W. Rethinking ALS: The FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas N.J., Roybon L. Harnessing the Potential of Human Pluripotent Stem Cell-Derived Motor Neurons for Drug Discovery in Amyotrophic Lateral Sclerosis: From the Clinic to the Laboratory and Back to the Patient. Front. Drug. Discov. 2021;1 doi: 10.3389/fddsv.2021.773424. [DOI] [Google Scholar]
- Leventoux N., Morimoto S., Hara K., Nakamura S., Ozawa F., Mitsuzawa S., Akiyama T., Nishiyama A., Suzuki N., Warita H., et al. Generation of an ALS human iPSC line KEIOi001-A from peripheral blood of a Charcot disease-affected patient carrying TARDBP p.N345K heterozygous SNP mutation. Stem Cell Res. 2020;47 doi: 10.1016/j.scr.2020.101896. [DOI] [PubMed] [Google Scholar]
- Lituma P.J., Woo E., O’hara B.F., Castillo P.E., Sibinga N.E.S., Nandi S. Altered synaptic connectivity and brain function in mice lacking microglial adapter protein Iba1. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2115539118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi Usaj M., Yeung C.H.L., Friesen H., Boone C., Andrews B.J. Single-cell image analysis to explore cell-to-cell heterogeneity in isogenic populations. Cell Syst. 2021;12:608–621. doi: 10.1016/j.cels.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S., Takahashi S., Ito D., Daté Y., Okada K., Kato C., Nakamura S., Ozawa F., Chyi C.M., Nishiyama A., et al. Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery. Cell Stem Cell. 2023;30:766–780.e9. doi: 10.1016/j.stem.2023.04.017. [DOI] [PubMed] [Google Scholar]
- Nakamura R., Tohnai G., Nakatochi M., Atsuta N., Watanabe H., Ito D., Katsuno M., Hirakawa A., Izumi Y., Morita M., et al. Genetic factors affecting survival in Japanese patients with sporadic amyotrophic lateral sclerosis: a genome-wide association study and verification in iPSC-derived motor neurons from patients. J. Neurol. Neurosurg. Psychiatry. 2023;94:816–824. doi: 10.1136/jnnp-2022-330851. [DOI] [PubMed] [Google Scholar]
- Naujock M., Stanslowsky N., Bufler S., Naumann M., Reinhardt P., Sterneckert J., Kefalakes E., Kassebaum C., Bursch F., Lojewski X., et al. 4-Aminopyridine Induced Activity Rescues Hypoexcitable Motor Neurons from Amyotrophic Lateral Sclerosis Patient-Derived Induced Pluripotent Stem Cells. Stem Cell. 2016;34:1563–1575. doi: 10.1002/stem.2354. [DOI] [PubMed] [Google Scholar]
- Nguyen T.B., Lac Q., Abdi L., Banerjee D., Deng Y., Zhang Y. Harshening stem cell research and precision medicine: The states of human pluripotent cells stem cell repository diversity, and racial and sex differences in transcriptomes. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.1071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G., Spitalieri P., Murdocca M., Centanini E., Sangiuolo F. Organoid factory: The recent role of the human induced pluripotent stem cells (hiPSCs) in precision medicine. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.1059579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H., Morimoto S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29:189–208. doi: 10.1016/j.stem.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Okano H., Yasuda D., Fujimori K., Morimoto S., Takahashi S. Ropinirole, a New ALS Drug Candidate Developed Using iPSCs. Trends Pharmacol. Sci. 2020;41:99–109. doi: 10.1016/j.tips.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S., Hong H., Nakagawa M., Tanabe K., Tezuka K.i., et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Palasantzas V.E.J.M., Tamargo-Rubio I., Le K., Slager J., Wijmenga C., Jonkers I.H., Kumar V., Fu J., Withoff S. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. 2023;39:268–284. doi: 10.1016/j.tig.2023.01.002. [DOI] [PubMed] [Google Scholar]
- Park J., Kim J., Lewy T., Rice C.M., Elemento O., Rendeiro A.F., Mason C.E. Spatial omics technologies at multimodal and single cell/subcellular level. Genome Biol. 2022;23:256. doi: 10.1186/s13059-022-02824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R., Duggal G., Love M.I., Irizarry R.A., Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radivojevic M., Rostedt Punga A. Functional imaging of conduction dynamics in cortical and spinal axons. Elife. 2023;12 doi: 10.7554/elife.86512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.T., Thorvaldsdóttir H., Thorvaldsdóttir H., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagner A., Briscoe J. Establishing neuronal diversity in the spinal cord: a time and a place. Development. 2019;146 doi: 10.1242/dev.182154. [DOI] [PubMed] [Google Scholar]
- Sances S., Bruijn L.I., Chandran S., Eggan K., Ho R., Klim J.R., Livesey M.R., Lowry E., Macklis J.D., Rushton D., et al. Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat. Neurosci. 2016;19:542–553. doi: 10.1038/nn.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli G., Lamorte G., Carlessi L., Ferrari D., Rota Nodari L., Binda E., Delia D., Vescovi A.L., De Filippis L. Mild Hypoxia Enhances Proliferation and Multipotency of Human Neural Stem Cells. PLoS One. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenke M., Prause H.C., Bergforth W., Przykopanski A., Rummel A., Klawonn F., Seeger B. Human-Relevant Sensitivity of iPSC-Derived Human Motor Neurons to BoNT/A1 and B1. Toxins. 2021;13:585. doi: 10.3390/toxins13080585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrimanesh M., Ding B. Generation and optimization of highly pure motor neurons from human induced pluripotent stem cells via lentiviral delivery of transcription factors. Am. J. Physiol. Cell Physiol. 2020;319:C771–C780. doi: 10.1152/ajpcell.00279.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.Y., Pfaff K.L., Davidow L.S., Sun C., Uozumi T., Yanagawa F., Yamazaki Y., Kiyota Y., Rubin L.L. Using Automated Live Cell Imaging to Reveal Early Changes during Human Motor Neuron Degeneration. eNeuro. 2018;5 doi: 10.1523/ENEURO.0001-18.2018. ENEURO.0001–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani N., Thompson L., Institute N., Australia Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci. 2014;8:293. doi: 10.3389/fncel.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takeda T., Iijima M., Shimizu Y., Yoshizawa H., Miyashiro M., Onizuka H., Yamamoto T., Nishiyama A., Suzuki N., Aoki M., et al. p.N345K mutation in TARDBP in a patient with familial amyotrophic lateral sclerosis: An autopsy case. Neuropathology. 2019;39:286–293. doi: 10.1111/neup.12559. [DOI] [PubMed] [Google Scholar]
- Tamaoka A., Arai M., Itokawa M., Arai T., Hasegawa M., Tsuchiya K., Takuma H., Tsuji H., Ishii A., Watanabe M., et al. TDP-43 M337V Mutation in Familial Amyotrophic Lateral Sclerosis in Japan. Intern. Med. 2010;49:331–334. doi: 10.2169/internalmedicine.49.2915. [DOI] [PubMed] [Google Scholar]
- Thams S., Lowry E.R., Larraufie M.H., Spiller K.J., Li H., Williams D.J., Hoang P., Jiang E., Williams L.A., Sandoe J., et al. A Stem Cell-Based Screening Platform Identifies Compounds that Desensitize Motor Neurons to Endoplasmic Reticulum Stress. Mol. Ther. 2019;27:87–101. doi: 10.1016/j.ymthe.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C., Rogelj B., Hortobágyi T., De Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., et al. Mutations in FUS, an RNA Processing Protein, Cause Familial Amyotrophic Lateral Sclerosis Type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger B.J., Kiskinis E., Mellin C., Wiskow O., Han S.S.W., Sandoe J., Perez N.P., Williams L.A., Lee S., Boulting G., et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E.R., Taherian Fard A., Mar J.C. Computational Methods for Single-Cell Imaging and Omics Data Integration. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R., Vaughn J.E. Choline acetyltransferase and NADPH diaphorase are co-expressed in rat spinal cord neurons. Neuroscience. 1994;63:1117–1124. doi: 10.1016/0306-4522(94)90577-0. [DOI] [PubMed] [Google Scholar]
- Xu H.J., Yao Y., Yao F., Chen J., Li M., Yang X., Li S., Lu F., Hu P., He S., et al. Generation of functional posterior spinal motor neurons from hPSCs-derived human spinal cord neural progenitor cells. Cell Regen. 2023;12:15. doi: 10.1186/s13619-023-00159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNA-seq data have been deposited at the Gene Expression Omnibus (GEO) database with accession number GSE229095 and are publicly available as of the date of publication.