Abstract
Purpose:
We evaluated biological effects of distinct local anesthetics on human adipose-derived mesenchymal stem cells when applied to reduce periprocedural pain during mesenchymal stem cell injections.
Methods and Materials:
Metabolic activity (MTS assay), viability (Live/Dead stain), and gene expression (quantitative real-time reverse-transcriptase polymerase chain reaction) were measured in mesenchymal stem cells incubated with various concentrations of lidocaine, ropivacaine, or bupivacaine during a 12-hr time course.
Results:
Cell viability and metabolic activity decreased in a dose, time, and substance-specific manner after exposure to lidocaine, ropivacaine, and bupivacaine, with ropivacaine being the least cytotoxic. Cell viability decreases after brief exposure (<1.5 hrs) at clinically relevant concentrations (eg, 8 mg/ml of lidocaine, 2.5 mg/ml of ropivacaine or bupivacaine). Mesenchymal stem cells exposed to local anesthetics change their expression of mRNA biomarkers for stress response (EGR1, EGR2), proliferation (MKI67, HIST2H4A), ECM (COL1A1, COL3A1), and cell surface marker (CD105).
Conclusions:
Local anesthetics are cytotoxic to clinical-grade human mesenchymal stem cells in a dose-, time-, and agent-dependent manner and change expression of ECM, proliferation, and cell surface markers. Lidocaine and bupivacaine are more cytotoxic than ropivacaine. Single-dose injections of local anesthetics may affect the biological properties of mesenchymal stem cells in vitro but may not affect the effective dose of MSCs in a clinical setting.
Keywords: Mesenchymal Stem Cells, Intra-articular Injection, Local Anesthetics, Cytoprotective Response
Biologics are popular experimental treatment modalities in rapidly developing field of regenerative medicine. The most commonly used treatments in musculoskeletal medicine are platelet-rich plasma, bone-marrow aspirate concentrate, microfragmented fat, mesenchymal stem cells (MSCs), and others.1,2 Injectable cell therapies may become an alternative treatment option when conservative treatment options have not been successful, and surgical treatment is not yet warranted or possible.3 Surgical interventions may contribute to patient’s burden by eliciting acute and chronic pain as well as postprocedure-related risks and require rehabilitation therapy.
There are several types of MSCs including those derived from bone marrow, adipose tissue, umbilical cord tissue, or other mesenchymal sources.4 Subcutaneous adipose tissue is an easily accessible source of adipose-derived MSCs with high yields.5–7 In addition, the procedure of fat tissue biopsy is considered quite safe and painless. Human MSCs can be rapidly expanded in vitro with small risk of clonal chromosomal and phenotypic changes.5,6 The expansion process leads to production of millions of MSCs during couple of weeks. Furthermore, zoonotic free MSCs cultured with human platelet lysate contribute to use of safe clinical grade–level MSCs.5
Mesenchymal stem cells are often promoted for their capacity to differentiate into several cell lineages.7 However, the primary benefit may lie in their trophic and immunomodulatory properties rather than directly replacing damaged or missing tissue. It is thought that bioactive molecules in sites of inflammation serve as local autologous medicine.4,8–10 Immunomodulation is mediated by anti-inflammatory cytokines such as interleukin (IL)-1Ra and IL-6. Although IL-6 is known to be proinflammatory, it has as well anti-inflammatory attributes that lead to increased expression of IL-1Ra and to decrease in expression of tumor necrosis factor α, IL-1, and IL-12.11
Current practice for therapeutic articular injections may include periarticular or intra-articular injection of local anesthetics for pain alleviation12 and therefore may have effect on transplantation of MSCs to the site of intervention. However, it is known that local anesthetics may be harmful to injected tissues, yet the mechanisms behind these effects are not well understood. Importantly, it has been shown that local anesthetics are cytotoxic to cartilage13–16 causing significant metabolic and molecular changes within the chondrocytes.16 However, in vivo chondrocytes are further protected in their natural environment because of their embedment by the extracellular matrix (ECM). Some studies suggest that MSCs can be even more sensitive to the effects of local anesthetics.13,17,18 Therefore, toxicity of local anesthetics to MSCs is an important factor to be considered when performing intra-articular injections. This effect may be emphasized in some types of anesthetics and especially when used in high concentrations.
In a recent review article, our group examined the state of the art in the field regarding the cellular effects of local anesthetics. This article revealed trends in the cytotoxic effects of different types of local anesthetics on MSCs.19 However, previously published studies differ in dosing and temporal aspects (eg, acute effects vs recovery after exposure) and did not fully resolve questions regarding relative cytotoxicity and/or the cytoprotective mechanisms that are activated to mitigate effects of local anesthetics. Therefore, the present study was designed to improve our understanding of risks associated with the use of local anesthetics in combination with MSCs and to recognize the implications it may have for clinical outcomes. In this study, we addressed which molecular changes occur in MSCs cultured in vitro in response to modifications in several variables, such as drug type, exposure time, and drug concentration for the three most commonly used local anesthetics (ie, lidocaine, ropivacaine, and bupivacaine) to clarify the potential effects that these pain-relieving agents may have on the viability and metabolic activity of a stem cell type that is actively used in clinical trials.
MATERIALS AND METHODS
Cell Isolation
Mesenchymal stem cells were previously isolated with institutional review board approval after obtaining written informed consents from the donors. Fat tissue biopsy was performed in three representative donors and collected tissue was consequently processed while following previously described protocols.5,6 Two of the donors were male, aged 41 and 54 yrs, and one donor was a female aged 32 yrs. Mesenchymal stem cells were propagated with platelet lysate containing zoonotic free media5 and were derived from the exact same donors that have been extensively characterized by RNA sequencing6,20 and for multilineage potential.21 These MSCs comply with rigorous CD cell surface marker validation based on stringent release criteria for MSCs used in ongoing clinical trials. Cells were expanded to the desired passage, expanded, frozen, and stored in liquid nitrogen for use in experiments. We used passages 6 or 7 in all our experiments.
Cell Culture and Treatment
Mesenchymal stem cells from each of the three donors were retrieved from liquid nitrogen and expanded in Advanced Minimum Essential Medium (Advanced MEM; Life Technologies, Grand Island, NY), supplemented with 5% human platelet lysate (PLTMax; Mill Creek Life Sciences, Rochester, MN), 2 mM Glutamax (Life Technologies, NY), 2 U/ml of heparin, and 1% Pen-Strep (100 U/ml of penicillin, 100 g/ml of streptomycin; Cellgro, Thermo Fisher Scientific, Dublin, Ireland). Mesenchymal stem cells remained in the incubator in 37°C in humidified atmosphere with 5% carbon dioxide for at least 48 hrs. At 80% confluency, MSCs were enzymatically detached and seeded at density 10 × 103/cm2 in 6- and 96-well plates. After 24 hrs of culture, MSCs were washed with phosphate buffered saline (Life Technologies, NY) and incubated with the following concentrations of lidocaine: 0.8% (8 mg/ml), 0.5% (5 mg/ml), 0.25% (2.5 mg/ml), and 0.125% (1.25 mg/ml); ropivacaine: 0.25% (2.5 mg/ml), 0.125% (1.25 mg/ml), 0.0625% (0.625 mg/ml), and 0.0313% (0.313 mg/ml); and bupivacaine: 0.25% (2.5 mg/ml), 0.125% (1.25 mg/ml), 0.0625% (0.625 mg/ml), and 0.0313% (0.313 mg/ml) for 0, 1.5, 3, 6, and 12 hrs. Reagents used in this study included the following: lidocaine 10 mg/ml in HCl (Hospira, Lake Forest, IL) and bupivacaine 2.5 mg/ml (Hospira, Lake Forest, IL), ropivacaine 2.5 mg/ml (Fresenius Kabi, Lake Zurich, IL), all in solution. Drugs were diluted in corresponding amounts of culture media to reach the desired concentrations. Control cells were cultured in culture media with the previously mentioned additives. After the treatment incubation, drug solutions were aspirated. Time-course and concentration-dependent experiments were performed with MSCs cell lines from three donors and using technical duplicates or triplicates.
Assessment of Viability and Metabolic Activity
To detect viability, proliferation and metabolic activity of MSCs, the cells from three donors were seeded in 96-well plates (Falcon) at a density of 10 × 103/cm2 cells, incubated in standard culture conditions for 24 hrs, and treated with four different concentrations of local anesthetics for 0, 1.5, 3, 6, and 12 hrs.
The viability profile of the MSCs exposed to the local anesthetics was determined using the Live/Dead assay kit with 2 fluorescent probes, Ethidium homodimer-1 and Calcein-AM (Molecular Probes, Thermo Fisher Scientific, Waltham, MA). The probes were diluted to 2 ul/ml PBS and 0.5 ul/ml PBS and added to the 96-well plates containing the MSCs previously treated with the local anesthetics. After 15-minute incubation, the MSCs were observed under a fluorescent microscope (ZeissAxioVert.A1) to determine viability. The proportions of live cells were assessed using fluorescence quantification at 465/450 nm (Infinite Pro 2000, Tecan, Austria) with the appropriate excitation filters.
To determine metabolic activity and proliferation after treatment with local anesthetics, cell proliferation was measured by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-car-boxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric assay (CellTiter 96 AQueous One Solution).22 At each time point, cells were incubated with 100 μl of MTS assay solution for approximately 1 hr. The total number of viable cells was assessed by absorbance measurement using a Spectra Max Plus Plate Reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 490 nm. After subtraction of the blank values, the results were normalized to the control cells.
Gene Expression Analysis
A real-time quantitative polymerase chain reaction analysis was performed with RNA from all three donors to assess mRNA expression after exposure to local anesthetics. Gene expression analysis was completed to observe expression levels of mRNA for proliferation markers, ECM production, stress-related and cytoprotective responses, stem cell surface markers, and hypoxia markers. The mRNA was isolated using Tri Reagent (Zymo Research, Irvine, CA) and Direct-zol RNA MiniPrep (Zymo Research Irvine, CA). RNA yield determined using Nanodrop 2000 (Thermo Scientific, Inc, Waltham, MA). Reverse transcription was performed to obtain cDNA using SuperScript III (Invitrogen, Carlsbad, CA). The cDNA was analyzed by real-time quantitative polymerase chain reaction (C1000 Touch Thermal Cycler; BioRad, Hercules, CA) using SYBR Green detection with specific primers. Primers’ sequences are given in Table 1. Results are normalized to GAPDH gene within each sample.
TABLE 1.
Primer sequences for quantitative PCR
| Gene | Primers, 5'-3' | Primers, 3'-5' |
|---|---|---|
|
|
|
|
| Forward | Reverse | |
|
| ||
| EGR1 | ACCCCTCTGTCTACTATTAAGGC | TGGGACTGGTAGCTGGTATTG |
| EGR2 | ATTCTGAGGCCTCGCAAGTA | GCTTATGCCCAGTGTGGATT |
| COL1A1 | GTAACAGCGGTGAACCTGG | CCTCGCTTTCCTTCCTCTCC |
| COL3A1 | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| HIF1a | TTCCAGTTACGTTCCTTCGATCA | CAGTTCCGCAAGCCCTGAAAGCG |
| HIF2a | GGACTTACACAGGTGGAG | TCTCAGGAATCTCCTCATG |
| HIF3a | TGGCAAGACCACAGGTCG | TACAGCACCTGGGTCTCCT |
| CD105 | TGCACTTGGCCTACAATTCCA | AGCTGCCCACTCAAGGATCT |
| GAPDH | ATGTTCGTCATGGGTGTGAA | TGTGGTCATGAGTCCTTCCA |
Statistical Analysis
Descriptive statistics were used for results obtained for all three donors to demonstrate the effects of lidocaine on human adipose-derived MSCs cell counts (ie, viability), mitochondrial activity, and function at various study time points after the exposure. Data were presented as the mean and standard deviation and analyzed by two-way analysis of variance (ANOVA) with Tukey’s post hoc test and nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons. Graph Pad Prism 7.05 was used for all analyses, and a P value of less than 0.05 was considered statistically significant.
RESULTS
Local Anesthetics Affect Viability of MSCs
Local anesthetics (LAs) have previously shown toxicity to MSCs; therefore, our study addresses biological effects of three types of LAs on MSCs derived from three clinically unremarkable donors without overt physiological conditions. We investigated whether LAs have dose or time dependent effects on the viability of MSCs. First, viability of cells and cell counts were determined using a Live/Dead staining after exposure to anesthetics during various incubation periods.
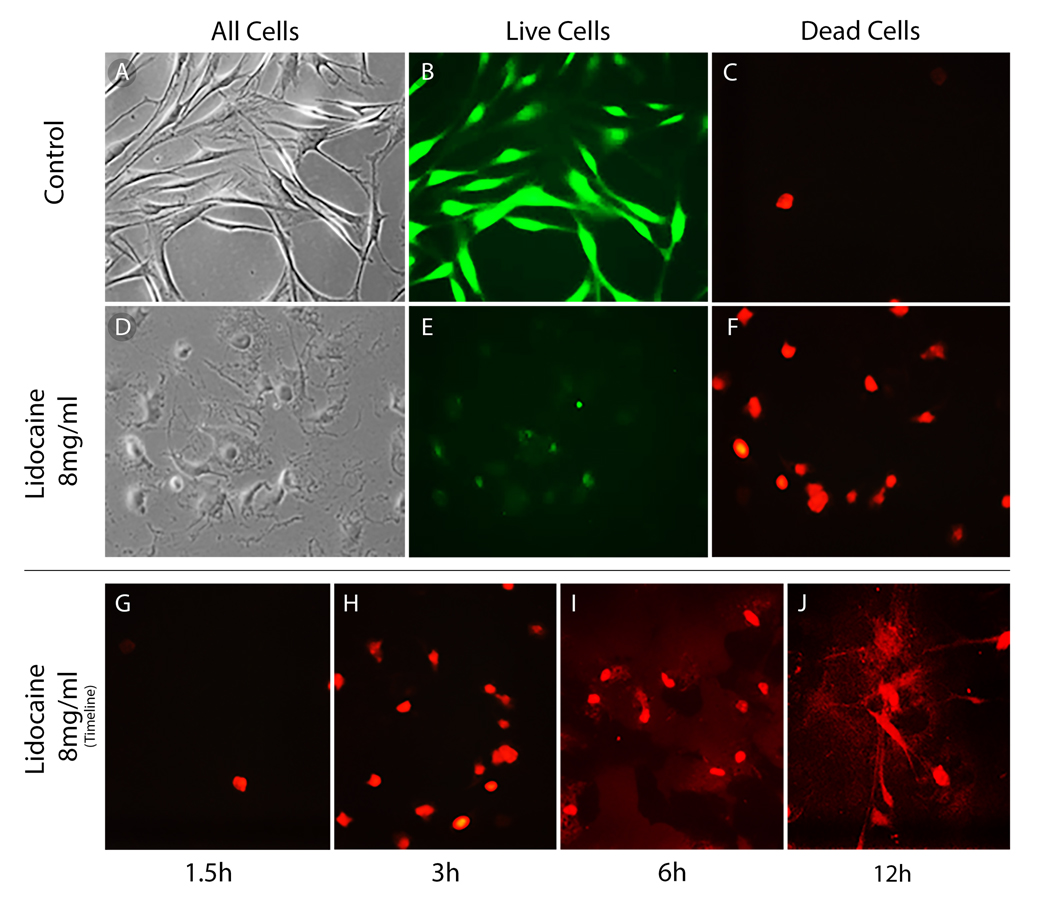
It has been previously shown that MSCs under effects of local anesthetics demonstrate features of apoptosis and necrosis.19 To evaluate effects of anesthetics on the MSCs morphology and viability the Live/Dead Assay was used. Mesenchymal stem cells were observed under a confocal microscope (Fig. 1). At 1.5 hrs, control cells were compared with the lidocaine treated cells. In the control cells, there were many live cells (Fig. 1B) and nearly no dead cells (Fig. 1C). However, with prolonged lidocaine exposure, viable cells were barely detected and amounts of dead cells increased markedly (Figs. 1G–J). Similar results were observed for cells exposed to bupivacaine and ropivacaine (data not shown). This observation showed that time-dependent toxic changes are triggered during exposure to lidocaine and other tested anesthetics.
FIGURE 1.
Lidocaine effects on human adipose-derived MSCs viability. Panels show control cells (A–C) and cell treated with lidocaine 8 mg/ml (D–F) stained with Live (B vs E) and Dead stain (C vs F) cells and cells with no stain under light microscopy (A) exposed during 1.5-hr period. Exposure to lidocaine 8 mg/ml and effects on viability are also shown during the periods: 1.5 (G), 3 (H), 6 (I), and 12 (J). The number of dead cells increases in a time-dependent manner.
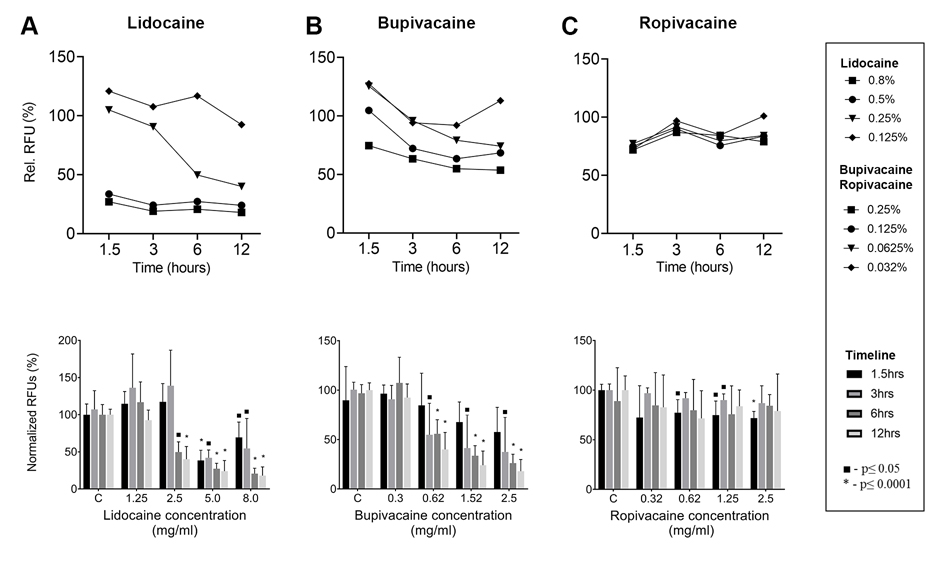
Quantification of viable cells in lidocaine of concentration 8 and 5 mg/ml showed significant decline at 1.5 hrs; however, it was less pronounced in the two lowest concentrations. At 3 hrs and longer time points, all cell numbers declined by less 50%, except for the lowest lidocaine concentration (Fig. 2A).
FIGURE 2.
Live/Dead assay quantification results show viability of the human adipose-derived MSCs. Panels A–C respectively illustrates relative fluorescent unit values immediately after exposure to local anesthetics of various concentrations to lidocaine (A), bupivacaine (B), and ropivacaine (C) at 1.5, 3, 6, and 12 hrs. Values represent relative fluorescence values measured at 465/450 nm. Statistical comparison of differences between controls and anesthetic exposed samples was performed with following significance cutoffs: ■ P < 0.05, *P < 0.0001, two-way ANOVA followed by Tukey’s post hoc test. C, control.
With bupivacaine treatment, time-dependent changes in viability were readily present at the first time point of 1.5 hrs and occurred mainly in the highest tested concentrations levels (2.5 and 1.25 mg/ml; Fig. 2B). Interestingly, number of cells seemed to be mildly increased in the 1.25 mg/ml and other lower concentrations at the shortest time point (1.5 hrs).
Ropivacaine effect on viability was significantly less detrimental to MSCs. Observable changes occurred mainly in the 1.5-hr time point (Fig. 2C).
Overall, time-dependent changes were observed and the viability of MSCs decreased to 75% at 1.5 hrs, regardless of the anesthetic type. This decline further continued in lidocaine and bupivacaine with prolonged exposure. The three highest concentrations of lidocaine (8, 5, and 2.5 mg/ml) and bupivacaine (2.5, 1.25, and 0.313 mg/ml) caused marked decrease of viability less than 50% if exposed for more than 6 hrs. Hence, only the lowest concentration of lidocaine (1.25 mg/ml) and bupivacaine (0.313 mg/ml) maintained persistent cell survival greater than 80% even after 12 hrs of exposure (Figs. 2A, B). The effects of ropivacaine seem to be more variable (Fig. 2C). The viability initially declined at 1.5 hrs in all concentrations to 75%; however, as the time progressed, there was no significant continuing decline. Interestingly, in some cases, the number of viable cells was even higher than that of the control cells. At 12 hrs of incubation, the least significant decline was found in the viability of MSCs treated with low concentrations (0.625 and 0.313 mg/ml) of bupivacaine or ropivacaine (Figs. 2B, C).
To sum up, the quantification of viable cells decreased as the concentration of local anesthetic increased in most time points (Fig. 2). The highest tested concentrations of lidocaine, ropivacaine, and bupivacaine (8, 2.5, and 2.5 mg/ml, respectively) are concentrations commonly used in clinical practice; therefore, it seems that local anesthetics may affect biological properties and dosing of MSCs in therapeutic settings with ropivacaine being the least cytotoxic.
Local Anesthetics Inhibit Mitochondrial Activity of MSCs
Local anesthetics–induced cytotoxicity may reduce metabolic activity and proliferation of MSCs because of the inhibitory effects on the cell cycle. To assess whether local anesthetics affects metabolism and proliferation of MSCs, we performed an assay to evaluate their mitochondrial activity. MTS assay was used in MSCs incubated with local anesthetics of the four concentrations that were previously used for viability testing (Fig. 3).
FIGURE 3.
MTS assay assessing metabolic activity of human adipose-derived MSC. Panels A–C illustrates values immediately after exposure to 0, 1.5, 3, 6, and 12 hrs of different concentrations and types of local anesthetic: lidocaine, bupivacaine, and ropivacaine, respectively. Values for the MTS assays represent absorbance values in percentage to control values measured at 490 nm. Statistical comparison of differences between controls and anesthetic exposed samples was performed with following significance cutoffs: ■ P < 0.05, *P < 0.0001, two-way ANOVA followed by Tukey’s post hoc test. C, control.
It was observed that MSCs treated with lidocaine and bupivacaine in the two highest concentrations (8 and 5 mg/ml for lidocaine and 2.5 and 1.25 mg/ml for bupivacaine) demonstrated significant decline of metabolic activity below 25% compared with the control values (Figs. 3A, B). Only the lowest concentrations (lidocaine 1.25 mg/ml, bupivacaine 0.313 mg/ml) had maintained their metabolic activity beyond 75% of control cells average.
The evaluation of time-dependent change showed that bupivacaine in the two highest concentrations (2.5 and 1.25 mg/ml) immediately decreased metabolic activity levels, which was maintained for 12 hrs. Lidocaine showed that the levels were declining with time, and in the two highest concentrations, they were less than 50% of the control values at 3 hrs. The lowest concentrations of lidocaine also showed a decline to approximately 80%, but the change was not time dependent. Most importantly, in ropivacaine (Fig. 3C), decrease of metabolic activity was less significant than in the other two anesthetics. The highest concentration (2.5 mg/ml) showed decline to 60% of baseline metabolic activity at 1.5 hrs and also slightly declined further to 50% at 12 hrs.
We can appreciate that lidocaine and bupivacaine caused more prominent continuous decline of mitochondrial activity and proliferative ability compared with ropivacaine. Ropivacaine has been shown to have more clear time- and concentration-dependent pattern of metabolic changes. Because of the severe metabolic activity compromise observed during the exposure of MSCs to local anesthetics in vitro, it was demonstrated that their toxicity is considerable in high concentrations. Therefore, diluted solutions of local anesthetics should be used in clinical practice and injected only after careful review of risks and benefits. Exposure time is an important variable that should be further tested in vivo to ensure elimination of the cytotoxic effects.
Effect of Local Anesthetics on mRNA Expression
Local anesthetics have been shown to be cytotoxic to MSCs. Therefore, it is anticipated that several bio cellular pathways are undergoing major changes during the exposure of MSCs to local anesthetics. We explored how lidocaine, bupivacaine, and ropivacaine can disturb cell functions and what changes may be imposed. Four different concentrations of each of the three local anesthetics were used to evaluate their effect on selected mRNA biomarkers after 1.5 hrs of exposure (Table 1). Molecular changes were also observed at 24 hrs after exposure to evaluate MSCs recovery. Quantitative real-time polymerase chain reaction analysis was performed to study biomarkers related to stress response and cytoprotection, proliferation, ECM production, hypoxia, as well as surface markers.
Cytoprotective markers that were studied were EGR1 and EGR2 (Figs. 4A, B). Early cytoprotective markers are usually expressed after a stressful event. Interestingly, the expression of EGR1 and EGR2 was significantly inhibited by all of the local anesthetic agents at 1.5 hrs in the highest concentrations of lidocaine and bupivacaine and mildly elevated in the lower concentrations (data not shown). In comparison, ropivacaine exhibited significantly increased levels in the highest concentrations of EGR1 and EGR2 genes associated with response to stress and cytoprotection (Fig. 4A). At 24 hrs after exposure, levels of these genes markedly decreased below baseline in all three anesthetics (Figs. 4A, B). However, levels of EGR1 in the second highest concentration of bupivacaine were significantly elevated (Fig. 4B; P < 0.001). For bupivacaine and lidocaine, our data were limited because of the difficulties during isolation of RNA from treated cells. The RNA yields in the highest levels of anesthetics were poor, which was caused by high cell mortality in the high concentrations.
FIGURE 4.
Effect of local anesthetics on human adipose-derived MSC proliferation and metabolism reflected by mRNA expression. The bar graphs in the panels show mRNA expression levels after lidocaine (8, 5, 2.5, 1.125 mg/ml), ropivacaine, and bupivacaine (2.5, 1.25, 0.625, 0.313 mg/ml) treatment of the following genes: early growth response genes/nerve growth factor-induced protein/transcription factor genes (A, B) and proliferation HIST2H4 (C). Gene expression was normalized to GAPDH. Values represent log 2 of the mean (with standard error of mean) of treatments that were sampled as biological duplicates (n = 6 total samples). *P < 0.05, one-way ANOVA followed by Tukey’s post hoc test. B, bupivacaine; L, lidocaine; R, ropivacaine.
It seems that stress-induced gene expression after exposure to anesthetics was increased in concentration-dependent manner in ropivacaine. Interestingly, in bupivacaine and lidocaine, similar elevation was observed only in the lower concentrations. Most of the stress-related responses subsided by 24 hrs.
In addition, to explore proliferation of MSCs after a local anesthetics application, changes in expression of proliferation markers HIST2H4A and MKI67 were observed (Figs. 4C, D). Interestingly, HIST2H4 marker of proliferation was increased in high concentrations of bupivacaine and ropivacaine and decreased in their lower concentrations. At 24 hrs, levels of HIST2H4 did not change significantly (data not shown). Marker MKI67 did not change immediately after exposure in lidocaine and ropivacaine (data not shown). However, in bupivacaine, the highest concentration caused significant decrease (P < 0.05) and lower concentration increase of MKI67 expression (Fig. 5A). At 24 hrs, expression of MKI67 in ropivacaine was elevated above baseline (4- to 8-fold change). Therefore, the proliferation capacity of MSCs after 1.5 hrs of exposure to LAs does not seem to be greatly impaired. Surprisingly, their proliferation may be even stimulated in lower concentrations and after 24 hrs of recovery.
FIGURE 5.
Effect of local anesthetics on human adipose-derived MSC proliferation and metabolism reflected by mRNA expression. The bar graphs in the panels show mRNA expression levels of the following genes after lidocaine (8, 5, 2.5, 1.125 mg/ml), ropivacaine, and bupivacaine (2.5, 1.25, 0.625, 0.313 mg/ml) treatment: proliferation MKI67 (A), ECM proteins and COL1 and COL3 (B). Gene expression was normalized to GAPDH. Values represent the mean (with standard error of mean) of treatments that were sampled as biological duplicates (n = 6 total samples). *P < 0.05, one-way ANOVA followed by Tukey’s post hoc test. B, bupivacaine; L, lidocaine; R, ropivacaine.
The production of ECM was evaluated in MSCs exposed to LAs, to detect their capabilities to produce extracellular tissue components (Fig. 5B). Exposure to any of the three anesthetics did not induce significant changes to COL1A1 and COL3A1 expression compared with baseline (data not shown). However, production of these ECM markers was markedly elevated at 24 hrs. Therefore, the exposure of MSCs to LAs is affecting production of ECM components, and their levels are elevated during the recovery period after the local anesthetics exposure.
Furthermore, we tested mRNA expression of surface marker CD105 specific for MSCs to observe for phenotypic changes induced by local anesthetics (Fig. 6A). Interestingly, the levels did not change significantly immediately after the exposure. However, the CD105 marker decreased significantly (P < 0.05) at the recovery period in most concentrations of all anesthetics. These results suggest that the all tested LAs may affect the phenotype of MSCs.
FIGURE 6.
Effect of local anesthetics on human adipose-derived MSC proliferation and metabolism reflected by mRNA expression. The bar graphs in the panels show mRNA expression levels after lidocaine (8, 5, 2.5, 1.125 mg/ml), ropivacaine, and bupivacaine (2.5, 1.25, 0.625, 0.313 mg/ml) treatment at time 1.5 hrs and 24 hrs after exposure of the following gene: stem cells marker CD105 (A). Gene expression was normalized to GAPDH. Values represent the mean (with standard error of mean) of treatments that were sampled as biological triplicates (n = 9 total samples). *P < 0.05, one-way ANOVA followed by Tukey’s post hoc test. B, bupivacaine; L, lidocaine; R, ropivacaine.
To examine whether MSCs exposure to LAs induced hypoxia-related factors expression, we evaluated expression of HIF1-α, HIF2-α/EPAS1, and HIF 3-α. No significant elevations of HIF 1-α, 2-α/EPAS1, and 3-α expression were observed (data not shown), indicating that exposure to local anesthesia does not induce significant hypoxic state.
DISCUSSION
The results of this study are relevant to stem cell therapy that has been introduced in multiple clinical trials as a mode of treatment for various orthopedic,23 skin,24,25 and other disorders.26–29 In particular, our results have bearing on cell therapies for a number of skeletal degenerative diseases that affect cartilaginous tissues in articular joints and spine.30–34 Cell therapies applications are typically accompanied by local anesthetics and other environmental factors that may potentially compromise viability during the preparation and delivery process. Our study establishes that local anesthetics have negative effects on stem cell viability and gene expression, consistent with previous studies.19,35 The findings in this study that local anesthetics may affect viability of stem cells complements other studies performed to address exposure to synovial fluid and hypoxia,36 various surface growth conditions,37,38 use of preservatives,39 iodine contrast toxicity,40 temperature changes,41 and diameter of needles in the injections.42 Together, these studies indicate that all procedures used during stem cell delivery in a therapeutic setting can alter the biological properties of stem cells and hence the efficacy and dosing in cell therapies.
Cell interactions with the local anesthetics substances most probably depend on their lipophilic or hydrophilic properties. Therefore, our studies included a comparison of the three most commonly applied anesthetics in the clinical practice including more lipophilic ropivacaine and bupivacaine. The experimental setting was designed to closely resemble the study on human chondrocytes. Lipophilic bupivacaine is more potent and hydrophilic than lidocaine and therefore more easily will reach intracellular space. Therefore, bupivacaine dosing can be lower to reach the desirable effect. The cytotoxic effects of lidocaine on MSCs viability and metabolic activity we observed in the present work are comparable with results from our previous studies.
Our current study investigated both short and extended exposure times (ie, 1.5–12 hrs) to local anesthetics in culture, because these parameters are relevant to the pharmacokinetics and clearance rates when anesthetic are applied intra-articularly. For example, lidocaine absorption in large joints occurs within the first hour after the application.43,44 Clearance of anesthetics may be accelerated preoperatively by fluid lavage, effusion presence, and inflammatory reactions.45 Because there are no published in vivo studies that have recorded the metabolism of local anesthetics in the knee joint and its bioavailability, we explored toxicity beyond the exposure times expected in clinical procedures. The most cytotoxic concentrations were usually detrimental immediately at the very first time point, whereas time-dependent changes were mainly observed in the lower concentrations of anesthetics. Cells with prolonged exposure (>1.5 hrs) and high concentrations of LAs (>8 mg/dl of lidocaine, 2.5 mg/dl of bupivacaine) seemed to exhibit compromised survival and metabolic activity, perhaps because of the induction of apoptosis, necrosis, and/or autophagy. All of these types of cell death were previously been shown to be associated with local anesthetic exposure.46,47 However, ropivacaine was shown to only slightly decrease the viability and metabolism of these cells. The results we presented here are consistent with findings in human chondrocytes14,15 and showed that ropivacaine demonstrates much milder cytotoxic effects than the other two anesthetics we tested.
From a molecular perspective, we showed that MSCs subjected to treatment by anesthetics express genes associated with responses to stress, including “early growth response” genes (eg, EGR1 and EGR2). The results suggest that MSCs undergo a cytoprotective response counteracting the cellular stress that emerges during lidocaine, ropivacaine, or bupivacaine exposure. In cells treated with ropivacaine, expression of cytoprotective genes is clearly concentration dependent and highly downregulated in the recovery period. Surprisingly, the most cytotoxic concentrations of lidocaine and bupivacaine as determined from viability testing do not cause a marked elevation of these genes, presumably because cells at these high concentrations perish and do not contribute to the RNA pool within our cell samples. Indeed, in some samples, cell death was nearly complete indicating that cell stress surpassed levels can be corrected by cytoprotective programs, thus triggering cell death pathways.
We evaluated mRNA levels of the well-known cancer histopathology-related Ki67 antigen MKI67 and the chromatin protein histone HIST2H4 that each represents very effective proliferation markers. Interestingly, proliferation markers levels increased slightly (fourfold) after a recovery period at 24 hrs. This result may suggest that a certain level of anesthetic toxicity may result in a rebound effect and lead to stimulation of the cell cycle progression. Similarly, production of ECM proteins seems to be unaffected immediately after exposure to anesthetics, but increases at 24-hr postexposure in all treatments we tested. For these reasons, we propose that although exposure to anesthetics impairs viability, the MSCs that survive are still able to proliferate and produce matrix after they recover.
Examination of mRNA levels for a representative MSC surface marker (CD105) revealed that short-term exposure (1.5 hrs) did not significantly affect expression of CD105 upon exposure to local anesthetics. However, the values of CD105 were decreased compared with control in all cell cultures treated with local anesthetics after a 1-day recovery period. Thus, short-term treatment may influence the long-term uncommitted differentiation phenotype (“stemness”) or function of human MSCs.
Based on the results from this study, MSCs may be more sensitive to the cytotoxicity of local anesthetics than chondrocytes that were investigated in a previous study. Our data suggest that lidocaine in the common concentrations was found to be more cytotoxic than ropivacaine and bupivacaine in MSCs. Ropivacaine was assessed to be the least cytotoxic from the three substances we tested and therefore may be the most optimal choice for use in the clinical setting, because it is least likely to affect biological dosing.
LIMITATIONS
This study was performed in vitro and therefore effects on cellular properties were obtained in a closed experimental environment. Cell culture systems are artificially supplemented with nutrients and lack key properties of the normal joint tissue niche such as growth factors or inflammatory markers. Furthermore, cell culture dishes do not permit dissipation of pain reducing agents into peripheral tissues resulting in retention of high concentrations for longer periods. Hence, the closed environmental setting of cells in culture does not fully recapitulate the time-dependent pharmacokinetics of these agents during therapeutic delivery. Our studies were based on studies with MSCs derived from donors undergoing elective lipoaspiration but were otherwise unremarkable, and we noted extensive donor-dependent variation for the different drugs, time points, and doses. This latter limitation influences the numerical responses and statistical significance of our results. This is compensated by the strength that our key observations (eg, relative cytotoxicity) seem to be valid, regardless of donor variability, which has potential ramifications for clinical expectations of the effects of local anesthetics.
CONCLUSIONS
We demonstrated that lidocaine, ropivacaine, and bupivacaine are all cytotoxic to human adipose-derived MSCs because they resulted in a decreased number of observable viable cells in a dose-, time-, and substance-specific manner. Local anesthetics affected the molecular mechanisms documented in mRNA expression levels changes. Understanding the effects of local anesthetics on these signaling systems may provide insights into novel strategies for mitigating cytotoxicity induced by anesthetics. In view of our findings, it is important to gain a working knowledge for medical providers about necessary caution in the selection of local anesthetics. Concentration and type of administered anesthetic during MSCs therapies may need to be considered to avoid compromising the viability and potency of MSCs in cell-based therapies for musculoskeletal degeneration or other degenerative conditions. However, another broad overall message that emerged from this work is that MSCs may also remain essentially viable under many typical conditions (except combination of high doses and exposure times). Based on these findings, rather than concerns with general cell viability and metabolic activity, future studies will now be able to focus on understanding how anesthetics can influence the potency of MSCs as “biological drugs” with anti-inflammatory and tissue-regenerative potential by assessment of changes in the secretome of MSCs. The latter will be key to understanding the potential effects (if any) of local anesthetics on the efficacy of MSCs in cell therapies.
What Is Known
Local anesthetics may be applied during stem cells injections to reduce patient discomfort. Previous studies have indicated that anesthetics such as lidocaine exhibit cytotoxicity for mesenchymal stem cells (MSCs) and chondrocytes.
What Is New
In the rapidly developing field of regenerative medicine including stem cells therapies, it is necessary to identify how the handling of cells may impact cell dosing during therapeutic delivery. Hence, we assessed how clinically relevant anesthetics affect stem cell viability and cellular functions. Our findings indicate that lidocaine and bupivacaine exhibit greater cytotoxicity than ropivacaine for MSCs. Anesthetics have impacted MSCs mRNA expression and therefore may affect their functions, showing a need for further investigations in future studies.
ACKNOWLEDGMENTS
The authors thank the members of our research group, including David Deyle, Roman Thaler, Amel Dudakovic, Daniela Galeano Garces, Holly Ryan, and Hai Nie, for general support of this project and stimulating discussions.
The authors also appreciate the generous philanthropic support of William H. and Karen J. Eby and the charitable foundation in their names.
This work was supported in part by the National Institutes of Health (Grant No. R01 AR049069, to AJvW).
Footnotes
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Steinert AF, Rackwitz L, Gilbert F, et al. : Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 2012;1:237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson S, Gerhardt M, Mandelbaum B: Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med 2008;1(3–4):165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo CH, Lee YG, Shin WH, et al. : Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial [published correction appears in Stem Cells. 2017 Jun;35(6):1651–1652]. Stem Cells 2014;32:1254–66 [DOI] [PubMed] [Google Scholar]
- 4.Murphy MB, Moncivais K, Caplan AI: Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crespo-Diaz R, Behfar A, Butler GW, et al. : Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant 2011;20:797–811 [DOI] [PubMed] [Google Scholar]
- 6.Dudakovic A, Camilleri E, Riester SM, et al. : High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem 2014;115:1816–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimble J, Guilak F: Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 2003;5:362–9 [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI, Correa D: The MSC: an injury drugstore. Cell Stem Cell 2011;9:11–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan AI, Dennis JE: Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–84 [DOI] [PubMed] [Google Scholar]
- 10.Guilak F, Awad HA, Fermor B, et al. : Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004;41(3–4):389–99 [PubMed] [Google Scholar]
- 11.Pers YM, Jorgensen C: Adipose derived stem cells for regenerative therapy in osteoarticular diseases. Horm Mol Biol Clin Invest 2016;28:113–20 [DOI] [PubMed] [Google Scholar]
- 12.Stephens MB, Beutler AI, O’Connor FG: Musculoskeletal injections: a review of the evidence. Am Fam Physician 2008;78:971–6 [PubMed] [Google Scholar]
- 13.Kreuz PC, Steinwachs M, Angele P: Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: a systematic review of the current literature. Knee Surg Sports Traumatol Arthrosc 2018;26:819–30 [DOI] [PubMed] [Google Scholar]
- 14.Dragoo JL, Braun HJ, Kim HJ, et al. : The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med 2012;40:794–9 [DOI] [PubMed] [Google Scholar]
- 15.Dragoo JL, Korotkova T, Kanwar R, et al. : The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med 2008;36:1484–8 [DOI] [PubMed] [Google Scholar]
- 16.Piper SL, Kramer JD, Kim HT, et al. : Effects of local anesthetics on articular cartilage. Am J Sports Med 2011;39:2245–53 [DOI] [PubMed] [Google Scholar]
- 17.Breu A, Eckl S, Zink W, et al. : Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy 2013;29:1676–84 [DOI] [PubMed] [Google Scholar]
- 18.Breu A, Scheidhammer I, Kujat R, et al. : Local anesthetic cytotoxicity on human mesenchymal stem cells during chondrogenic differentiation. Knee Surg Sports Traumatol Arthrosc 2015;23:937–45 [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Smith J, Nie H, et al. : Cytotoxicity of local anesthetics in mesenchymal stem cells. Am J Phys Med Rehabil 2018;97:50–5 [DOI] [PubMed] [Google Scholar]
- 20.Camilleri ET, Gustafson MP, Dudakovic A, et al. : Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther 2016;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudakovic A, Camilleri ET, Lewallen EA, et al. : Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. J Cell Physiol 2015;230:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denizot F, Lang R: Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89:271–7 [DOI] [PubMed] [Google Scholar]
- 23.Iijima H, Isho T, Kuroki H, et al. : Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med 2018;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klar AS, Zimoch J, Biedermann T: Skin tissue engineering: application of adipose-derived stem cells. Biomed Res Int 2017;2017:9747010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf DA, Beeson W, Rachel JD, et al. : Mesothelial stem cells and stromal vascular fraction for skin rejuvenation. Facial Plast Surg Clin North Am 2018;26:513–32 [DOI] [PubMed] [Google Scholar]
- 26.Bateman ME, Strong AL, Gimble JM, et al. : Concise review: using fat to fight disease: a systematic review of nonhomologous adipose-derived stromal/stem cell therapies. Stem Cells (Dayton, Ohio) 2018;36:1311–28 [DOI] [PubMed] [Google Scholar]
- 27.Amorin B, Alegretti AP, Valim V, et al. : Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Hum Cell 2014;27:137–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare JM, Fishman JE, Gerstenblith G, et al. : Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON Randomized Trial. JAMA 2012;308:2369–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peired AJ, Sisti A, Romagnani P: Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int 2016;2016:4798639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riester SM, Lin Y, Wang W, et al. : RNA sequencing identifies gene regulatory networks controlling extracellular matrix synthesis in intervertebral disk tissues. J Orthop Res 2018;36:1356–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Lewallen EA, Camilleri ET, et al. : RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J Orthop Res 2016;34:1950–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewallen EA, Bonin CA, Li X, et al. : The synovial microenvironment of osteoarthritic joints alters RNA-seq expression profiles of human primary articular chondrocytes. Gene 2016;591:456–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riester SM, Denbeigh JM, Lin Y, et al. : Safety studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a phase I clinical trial. Stem Cells Transl Med 2017;6:910–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Perez-Terzic CM, Smith J, et al. : Efficacy of intervertebral disc regeneration with stem cells - a systematic review and meta-analysis of animal controlled trials. Gene 2015;564:1–8 [DOI] [PubMed] [Google Scholar]
- 35.Nie H, Kubrova E, Wu T, et al. : Effect of lidocaine on viability and gene expression of human adipose-derived mesenchymal stem cells: an in vitro study. PM R 2019;11:1218–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galeano-Garces C, Camilleri ET, Riester SM, et al. : Molecular validation of chondrogenic differentiation and hypoxia responsiveness of platelet-lysate expanded adipose tissue-derived human mesenchymal stromal cells. Cartilage 2017;8:283–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Y, Denbeigh JM, Camilleri ET, et al. : Extracellular matrix protein production in human adipose-derived mesenchymal stem cells on three-dimensional polycaprolactone (PCL) scaffolds responds to GDF5 or FGF2. Gene Rep 2018;10:149–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewallen EA, Jones DL, Dudakovic A, et al. : Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene 2016;581:95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollock K, Samsonraj RM, Dudakovic A, et al. : Improved post-thaw function and epigenetic changes in mesenchymal stromal cells cryopreserved using multicomponent osmolyte solutions. Stem Cells Dev 2017;26:828–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu T, Nie H, Dietz AB, et al. : Cytotoxic effects of nonionic iodinated contrast agent on human adipose-derived mesenchymal stem cells. PM R 2018. doi: 10.1016/j.pmrj.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubrova E, Qu W, Galvan ML, et al. : Hypothermia and nutrient deprivation alter viability of human adipose-derived mesenchymal stem cells. Gene 2020;722:144058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onishi K, Jones DL, Riester SM, et al. : Human adipose-derived mesenchymal stromal/stem cells remain viable and metabolically active following needle passage. PM R 2016;8:844–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz JA, Kaeding CS, Hill JR, et al. : The pharmacokinetics of bupivacaine when injected intra-articularly after knee arthroscopy. Anesth Analg 1988;67:872–5 [PubMed] [Google Scholar]
- 44.Convery PN, Milligan KR, Quinn P, et al. : Efficacy and uptake of ropivacaine and bupivacaine after single intra-articular injection in the knee joint. Br J Anaesth 2001;87:570–6 [DOI] [PubMed] [Google Scholar]
- 45.Stopka SS, Wilson GL, Pearsall AW: Dilution effect of intra-articular injection administered after knee arthroscopy. J Surg Orthop Adv 2015;24:209–12 [PubMed] [Google Scholar]
- 46.Johnson ME, Uhl CB, Spittler KH, et al. : Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology 2004;101:1184–94 [DOI] [PubMed] [Google Scholar]
- 47.Zhuang H, Hu D, Singer D, et al. : Local anesthetics induce autophagy in young permanent tooth pulp cells. Cell Death Discov 2015;1:15024. [DOI] [PMC free article] [PubMed] [Google Scholar]