Abstract
Background
The understanding of how tuberculosis is transmitted can be improved by combining DNA fingerprinting of Mycobacterium tuberculosis with conventional epidemiologic methods. We used such techniques to determine the predictors of clustering of identical isolates from tuberculosis patients in Vancouver.
Methods
We used the restriction fragment length polymorphism (RFLP) technique and, if necessary, spoligotyping to determine DNA patterns of M. tuberculosis isolates from all patients with newly diagnosed tuberculosis in Greater Vancouver reported to the Division of Tuberculosis Control from January 1995 to March 1999. Isolates were considered to be part of a cluster if they had an identical DNA pattern. We also collected demographic and epidemiologic data. Predictors associated with being in a cluster were analyzed in a multivariate logistic regression model.
Results
Isolates from 793 patients (430 men) were identified; 137 (17.3%) were considered to be in clusters. After adjustment for multiple potential predictors, we found that the following patients were more likely to be part of a cluster: Canadian-born Aboriginals (v. foreign-born patients) (adjusted odds ratio [OR] 6.0, 95% confidence interval [CI] 3.0–11.7), Canadian-born non-Aboriginals (v. foreign-born patients) (adjusted OR 3.6, 95% CI 2.1–6.3), and injection drug users (v. patients who did not inject drugs) (adjusted OR 3.9, 95% CI 1.9–8.1). Patients with a prior history of tuberculosis were less likely to be part of a cluster than were patients with no history of tuberculosis (adjusted OR 0.3, 95% CI 0.1–0.8).
Interpretation
Our findings indicate the need to target groups at high risk of tuberculosis more aggressively to prevent transmission and to treat latent infection. DNA fingerprinting may be a useful adjunct to conventional epidemiologic methods to monitor the transmission of tuberculosis in an innter-city setting.
Tuberculosis is a leading cause of illness and death around the world.1 A convergence of factors, including HIV-related disease,2,3 increased immigration from countries with a high prevalence of tuberculosis and a deteriorating public health system,4 especially in Eastern Europe,5 have set the stage for increased transmission of tuberculosis. The combination of DNA fingerprinting of Mycobacterium tuberculosis and conventional epidemiologic methods has improved our understanding of the transmission of tuberculosis.6,7,8,9,10 We used DNA fingerprinting in a population-based study of tuberculosis to determine predictors associated with being in a cluster (having M. tuberculosis isolates with an identical DNA pattern) in order to better understand the transmission of tuberculosis in Greater Vancouver.
Methods
We identified all cases of newly diagnosed culture-positive tuberculosis in Greater Vancouver reported to the Division of Tuberculosis Control of the BC Centre for Disease Control from January 1995 to March 1999. All mycobacteriological testing in British Columbia is performed at the BC Centre for Disease Control Laboratory Services. Mycobacterium species are identified by means of standard biochemical tests and RNA hybridization. Positive cultures were collected and forwarded to the study laboratory in Edmonton for DNA fingerprinting using the standard restriction fragment length polymorphism (RFLP) typing method with the insertion sequence IS6110 as a probe.6
In brief, the RFLP technique consists of cutting the DNA with a restriction enzyme, separating the DNA by means of gel electrophoresis, transferring the DNA to a membrane by means of blotting, and identifying sequences of interest by means of hybridization to a labelled probe. The genetic characteristics identified by RFLP can help to determine the distribution of isolates during outbreaks, to examine cross-contamination in clinical isolates and to evaluate whether the occurrence of disease in a previously treated patient is the result of a new infection or reactivation of latent tuberculosis.
We used computer-assisted DNA pattern recognition to analyze the isolates. A cluster was defined as 2 or more isolates with an identical DNA pattern (6 or more copies of IS6110 identified by means of RFLP or, if fewer than 6 copies, the same DNA pattern identified by means of spoligotyping11). The first case diagnosed was assumed to be the index case for the cluster. Standard methods were used to exclude clusters due to apparent cross-contamination.7
In addition to the laboratory evaluation of the isolates, we collected epidemiologic, demographic and clinical data, using standardized data collection sheets, from a computerized database maintained by the Division of Tuberculosis Control. Information gathered included risk factors for tuberculosis, prior history of tuberculosis, contact history, employment status, site of disease, previous bacille Calmette–Guérin (BCG) vaccination and tuberculin skin test results. Ethnic background was determined according to country of birth.
For statistical analysis we divided the sample into 2 groups: clustered isolates (as described above) and nonclustered isolates (those with a unique RFLP pattern or with fewer than 6 copies of IS6110 but with a different pattern identified by means of spoligotyping). Descriptive statistics, including cross-tabulations of demographic and clinical characteristics were computed. The χ2 test, or the Fisher's exact test when applicable, was used in a univariate analysis to assess risk factors associated with clustering. Predictors significantly associated with clustering (p < 0.05) were included in a multivariate logistic regression model, with being in a cluster or not being in a cluster as the dependent variables.
The study protocol was approved by the University of British Columbia Human Ethics Committee.
Results
From January 1995 to March 1999 a total of 806 cases of tuberculosis were diagnosed in Greater Vancouver. We excluded 8 duplicate entries and 5 cases because the patients were seen before 1995. Thus, the total sample comprised 793 cases. Of these, 137 isolates (17.3%) were grouped into 46 clusters according to their RFLP patterns. In 5 of the 46 clusters a total of 11 isolates (8.0% of the clustered isolates) had fewer than 6 identical bands identified using the RFLP technique, but the bands were found to be the same by means of spoligotyping. In the 41 remaining clusters, all 126 isolates (92% of the clustered isolates) had more than 6 identical bands, as determined by RFLP typing.
The demographic and clinical characteristics of the patients are shown in Table 1. Table 2 shows the maximum likelihood estimates from the multivariate analysis. Because age was not found to be linearly associated with clustering in the multivariate analysis, a stratified analysis was conducted for isolates from patients 60 years and younger and those from patients over 60.
Table 1
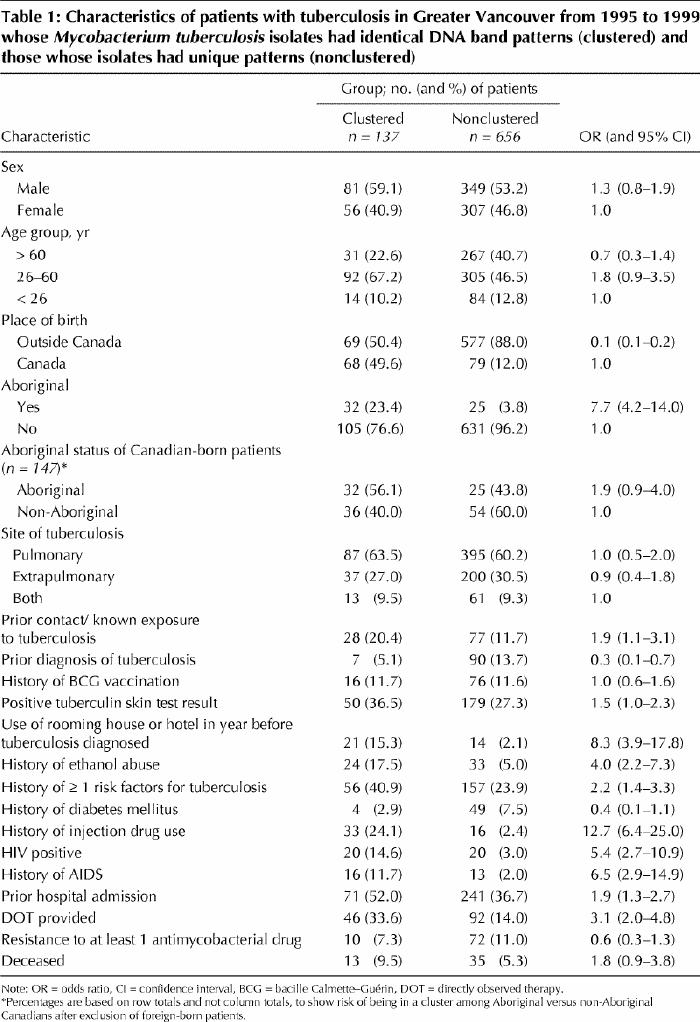
Table 2
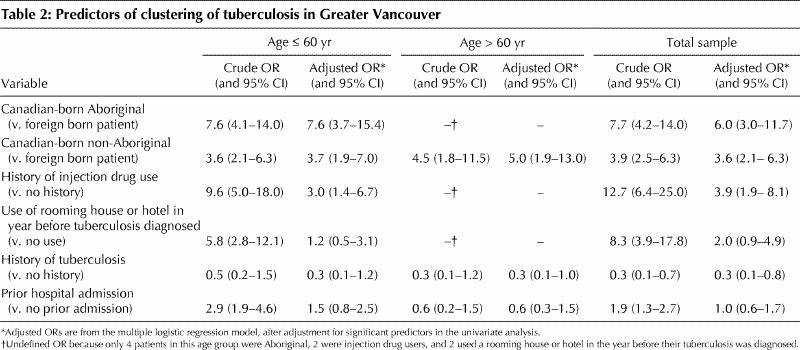
The multivariate analysis showed that, among patients 60 years and younger, the strongest predictor of clustering was being a Canadian-born Aboriginal (v. foreign-born person) (adjusted odds ratio [OR] 7.6, 95% confidence interval [CI] 3.7–15.4); the next strongest predictors were being a Canadian-born non-Aboriginal (v. foreign-born person) (adjusted OR 3.7, 95% CI 1.9–7.0) and being an injection drug user (v. not being an injection drug user) (adjusted OR 3.0, 95% CI 1.4–6.7). Among patients over 60 years, the significant predictor associated with clustering was being a Canadian-born non-Aboriginal (v. foreign-born person) (adjusted OR 5.0, 95% CI 1.9–13.0). In this age group, there were only 2 injection drug users, 4 Aboriginals and 2 patients who had used a rooming house or hotel in the year before diagnosis; therefore, the estimates of these variables were undefined. Tests for interaction were conducted using sex, age and the 3 variables found to be significant after adjustment (Canadian-born Aboriginal and non-Aboriginal, and injection drug user), but no significant interactions were found in the total sample or in either of the 2 age groups.
Of the 137 patients whose isolates were in clusters, 11 (8.0%) identified each other as contacts by conventional contact-tracing means.
Interpretation
In this study we used molecular epidemiology11 to determine the predictors associated with clustering of tuberculosis cases in Greater Vancouver. Only 8% of the cases linked by RFLP typing in our study were known to each other using traditional contact-tracing methods.
We found that 137 (17.3%) of the patients with newly diagnosed culture-positive tuberculosis had isolates belonging to clusters. A similar proportion (19%) was found in San Francisco from 1991 to 1997,10 and higher proportions have been reported in other studies.6,7,8 Our multivariate analysis confirmed that the most important independent predictors of being in a cluster in Vancouver were being a Canadian-born Aboriginal, being a Canadian-born non-Aboriginal and being an injection drug user. Having a prior history of tuberculosis was protective against clustering.
Compared with non-clustered isolates, clustered isolates were 6 times more likely to be from Aboriginal patients. This risk increased to eightfold among Aboriginals 60 years and younger, which suggests that younger Aboriginal people play an important role in the transmission of tuberculosis in Greater Vancouver. Of the Canadian-born non-Aboriginal patients, on the other hand, clustering was most common among those over 60, which suggests that in this age group non-Aboriginal people are playing an important role in the transmission of tuberculosis in Vancouver. In addition to social factors, preliminary data suggest that Aboriginal people in Canada,12 similar to other indigenous peoples,13 have a gene deletion for NRAMP1, which may predispose them to acquiring active tuberculosis.
HIV seropositivity6 and a history of AIDS7 have been associated with being in a cluster in previous studies from the United States. The results of our univariate analysis indicated that HIV seropositivity was associated with clustering, but this association was not found after adjustment in the multivariate analysis. Injection drug use was a strong predictor of clustering in our study. Interestingly, despite 2 recent reports of apparent reinfection with a different strain of M. tuberculosis,14,15 we found no cases of reinfection by a different organism in our study.
We found no association between drug resistance and clustering, as has been previously reported.6,9 Not surprisingly, people with pulmonary tuberculosis were more likely to be found among the clustered cases than among the nonclustered cases. Analyzing documented risk factors for tuberculosis showed that people with a history of ethanol abuse or at least one medical condition predisposing to tuberculosis were at increased risk of being in a cluster. Death from tuberculosis as a risk factor for clustering is likely based on the greater likelihood of clustering among HIV-positive patients and the general socioeconomic status of our marginalized inner-city population.
In summary, we have comprehensively described the molecular epidemiology of tuberculosis in Greater Vancouver, identifying significant disease transmission among defined high-risk groups. The data help to meet the challenge of achieving tuberculosis control in an inner-city population16 by identifying groups at risk for recent transmission and those that might benefit from treatment of latent disease. The success of any intervention can be monitored by using molecular epidemiology to identify patterns of transmission on a regular basis.
β See related articles pages 353 and 355
Acknowledgments
We thank the nurses, outreach workers and laboratory technicians who facilitated the collection of epidemiological data and processing of samples for this study.
Footnotes
This article has been peer reviewed.
Contributors: Dr. FitzGerald devised the study protocol and obtained funding; with Dr. Hernández-Garduño, he wrote the first draft of the paper. Drs FitzGerald, Elwood, Hernández-Garduño and Wang coordinated the collection of the epidemiological data. Drs. Black and Rodrigues supervised the culture and shipment of specimens to Dr. Kunimoto's laboratory, where Dr. Kunimoto coordinated and completed the molecular work. Mr. Mak reviewed the manuscript and assissted in the analysis of the data. Dr. Hernández-Garduño performed the statistical analysis. All authors contributed to completion of the final manuscript.
This project was funded in part by the Medical Services Branch of Health Canada, the BC Lung Association and the MRC/PMAC. Dr. FitzGerald is the recipient of a Vancouver General Hospital Scientist Award and a CIHR/BC Lung Scientist Award.
Competing interests: None declared.
Correspondence to: Dr. J. Mark FitzGerald, Center for Clinical Epidemiology and Evaluation Vancouver General Hospital Research Pavilion, 703–828 W 10th Ave., Vancouver BC V5Z 1L8; markf@interchange.ubc.ca
References
- 1.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull World Health Organ 1992;70:149-59. [PMC free article] [PubMed]
- 2.FitzGerald JM, Houston S. Tuberculosis: 8. The disease in association with HIV infection. CMAJ 1999;161(1):47-51. [PMC free article] [PubMed]
- 3.Barnes PF, Block AB, Davidson PT, Snider DE Jr. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 1991;324:1644-50. [DOI] [PubMed]
- 4.Brudney K, Dobkin J. Resurgent tuberculosis in New York City: human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis 1991;144:745-9. [DOI] [PubMed]
- 5.Grange JM, Zumla A. Paradox of the global emergency of tuberculosis. Lancet 1999;353:996. [DOI] [PubMed]
- 6.Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiology methods. N Engl J Med 1994;330: 1710-6. [DOI] [PubMed]
- 7.Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med 1994;330:1703-9. [DOI] [PubMed]
- 8.Gutierrez MC, Vincent V, Aubert D, Bizet J, Gaillot O, Lebrun L, et al. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J Clin Microbiol 1998;36:486-92. [DOI] [PMC free article] [PubMed]
- 9.Fandinho FC, Kritski AL, Hofer C, Junior Conde H, Ferreira RM, Saad MH, et al. RFLP patterns and risk factors for recent tuberculosis transmission among hospitalized tuberculosis patients in Rio de Janeiro Brazil. Trans R Soc Trop Med Hyg 2000;94:271-5. [DOI] [PubMed]
- 10.Jasmer RM, Hahn JA, Small PM, Daley CL, Behr MA, Moss AR, et al. A molecular epidemiologic analysis of tuberculosis trends in San Francisco 1991–1997. Ann Intern Med 1999:30:971-8. [DOI] [PubMed]
- 11.Van Soolingen D. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J Intern Med 2001:249:1-26. [DOI] [PubMed]
- 12.Greenwood CMT, Fujiwara TM, Boothroyd LJ, Miller MA, Frappier D, Fanning EA, et al. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large Aboriginal Canadian family. Am J Hum Genet 2000;67:405-16. [DOI] [PMC free article] [PubMed]
- 13.Bellamy R, Ruwende C, Corrah T, McAdam K, Whittle HC, Hill AVS. Variations in the NRAMP1 gene susceptibility to tuberculosis in West Africans. N Engl J Med 1998;338:640-4. [DOI] [PubMed]
- 14.Caminero JA, Pena MJ, Campos-Herrero MI, Rodriguez JC, Afonso O, Martin C, et al Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am J Respir Crit Care Med 2001;163:717-20. [DOI] [PubMed]
- 15.Van Rie A, Warren R, Richardson M, Vector TC, Gie RP, Enarson DA, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment N Engl J Med 1999;341:1174-9. [DOI] [PubMed]
- 16.FitzGerald JM. Optimizing tuberculosis control in the inner city. CMAJ 1999;160(6):821-2. [PMC free article] [PubMed]


