Abstract
Pericytes are multifunctional mural cells that surround the abluminal wall of endothelial cells and are associated with vascular development, vascular permeability, and angiogenesis. Additionally, pericytes demonstrate stem cell-like properties and contribute to neuroinflammatory processes. Pericytes have been extensively studied in the central nervous system. However, specific mechanisms underlying its involvement in various physiological and pathological conditions, especially in erectile dysfunction (ED), remain poorly understood. Advancements in in vitro and in vitro techniques, such as single-cell RNA sequencing, are expanding our understanding of pericytes. Recent studies have shown that pericyte dysfunction is considered an important factor in the pathogenesis of vascular and neurological ED. Therefore, this study aims to analyze the specific role of pericytes in ED, focusing on diabetic and neurogenic ED. This article provides a comprehensive review of research findings on PubMed from 2000 to 2023, concerning pericyte dysfunction in the process of ED, offering valuable insights, and suggesting directions for further research.
Keywords: angiogenesis, blood–brain barrier, erectile dysfunction, nerve regeneration, pericyte, stem cell
INTRODUCTION
The penis is a highly neurovascularized organ consisting of various types of soft-tissue structures and diverse cell populations. These cells are involved in essential physiological processes such as gas exchange, immunity, inflammation, detoxification, and tissue repair.1,2 Recent advancements in single-cell analysis technology, as evidenced by a recent study,3 have shown that the cell types within penile erectile tissue primarily include endothelial cells, fibroblast, pericytes, smooth muscle cells, Schwann cells, immune cells, and mesenchymal cells. Despite extensive research on most cell types in penile tissue, investigations into pericytes remain in its nascent stages.4 A number of vascular and neurogenic factors, such as diabetes, vascular disease, prostate problems, and neurogenic disorders, cause erectile dysfunction (ED) in most men.5 Dysfunction of pericytes in penile tissue may be implicated in these conditions.
Pericytes are versatile mural cells that wrap around the abluminal wall of endothelial cells, regulating vascular stability through direct physical contact and paracrine signals.6 Their morphology, distribution, density, and molecular fingerprint vary significantly across organs and vascular beds.7 Pericytes promote endothelial cell survival and migration, which contribute to angiogenesis.8 In the central nervous system (CNS), pericytes collaborate with astrocytes to maintain the activity of the blood–brain barrier (BBB).9 They also regulate blood flow at capillary junctions10 and promote neuroinflammatory processes.11 In addition, pericyte dysfunction is implicated in the progression of vascular diseases such as Alzheimer’s disease.12 Despite extensive research on pericytes in the CNS, investigation into their role in the penile tissue remains in its early stages, with the detailed mechanism still poorly understood.
Therefore, this review aims to evaluate current research on pericytes in penile tissue and explore the potential mechanisms through which pericytes regulate penile angiogenesis and nerve regeneration in different ED models.
PENILE PERICYTE
After conducting a literature review on PubMed (searching keywords “penile” and “pericytes” in May 2023), we found only 29 relevant articles. Penile pericytes were first mentioned in 1981 by Rao et al.13 in a case of angiolymphoid hyperplasia with penile eosinophilia, demonstrating significant proliferation of swollen endothelial cells and pericytes. Following this, until 2015, Yin et al.4 became the first to establish the specific distribution of pericytes in penile tissue and elucidate their pivotal role in the process of penile erection. Using both two-dimensional (2D) and three-dimensional (3D) imaging techniques, they observed abundant distribution of pericytes in the subtunical and dorsal nerve bundle regions. They also successfully isolated pericytes from mouse penis and human corpus cavernosum tissues and evaluated their function under pathological conditions in vitro and in vivo. Their findings demonstrated that pericytes can reduce cavernous body permeability and restore erectile function.4,14 However, they did not explicitly elucidate the mechanism underlying this phenomenon. Pericytes play a role in the BBB by modulating BBB-specific gene expression patterns in endothelial cells and inducing polarization of perivascular astrocytes in the CNS.15 Therefore, it can be speculated that pericytes may restore vascular stability and reduce permeability by regulating the expression of endothelial cell-related genes and proteins. Further related research will provide valuable insights into the underlying mechanism through which pericytes contribute to the process of erection.
Pericyte markers
Pericytes demonstrate diverse embryonic origins across different organs, leading to the identification of various pericyte subtypes. Therefore, the selection of pericyte markers should be classified according to specific organ contexts.16 In our study, we performed a screening of pericyte markers, presenting detailed experimental results and organizing them based on organ specificity (Table 1).4,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 The most representative molecular markers of pericytes include platelet-derived growth factor receptor beta (PDGFRβ), neural/glial antigen 2 (NG2), melanoma cell adhesion molecule (CD146), alpha-smooth muscle actin (α-SMA), regulator of G protein signaling 5 (RGS5), and desmin.38 These markers are widely expressed in pericytes across various organs (Table 1). However, most of these markers are also expressed by other cell types, such as oligodendrocyte precursor cells, vascular smooth muscle cells, and fibroblasts.38 In addition, many markers have been identified with specific expression patterns in particular organs. For example, aminopeptidase N (CD13) is found exclusively in cerebral pericytes, owing to its role in neurotransmitter metabolism within the BBB.39 Additionally, studies have confirmed pericyte-specific markers in various organs. For example, He et al.18 and Ayloo et al.40 demonstrated the specific expression of vitronectin18,40 and interferon-induced transmembrane protein 1 (Ifitm1)18 in mouse brain tissue. Single-cell sequencing analysis performed by Baek et al.22 revealed genes that are differentially expressed in pericytes across different organs. These include potassium two-pore domain channel subfamily K member 3 (Kcnk3) in the lung, regulator of G protein signaling 4 (Rgs4) in the heart, Purkinje cell protein 4 like 1 (Pcp4l1) in the bladder, myosin heavy chain 11 (Myh11), and potassium voltage-gated channel subfamily A member 5 (Kcna5) in the kidney.22 Recently, Bae et al.37 showed that limb bud-heart (Lbh) serves as a distinctive marker, enabling clear differentiation of pericytes from other cell types, such as smooth muscle cells and fibroblasts in both mouse and human cavernous tissues. Furthermore, as single-cell analysis technology continues to advance, many pericyte markers have been identified. However, research into the existence and function of these markers is still in its early stages, particularly concerning their variation under different physiological and pathological conditions. Given that pericyte phenotype can change accordingly, accompanied by alterations in specific gene expression, it becomes imperative to identify a multitude of pericyte-specific markers and explore the associated signaling pathways. Such endeavors will undoubtedly enhance our understanding of the roles played by pericytes in angiogenesis and nerve regeneration.
Table 1.
Markers for pericytes in different organs
| Organs | Traditional pericyte marker | Specific pericyte marker | Reference |
|---|---|---|---|
| Brain | PDGFRβ, NG2, CD146, α-SMA, RGS5, Desmin, CD13, Vitronectin, and Ifitm1 | CD13, vitronectin, and Ifitm1 | 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 |
| Lung | PDGFRβ, NG2, CD146, α-SMA, RGS5, Desmin, PDGFRα, Vimentin, Higd1b, Kcnk3, Cox4i2, Gucy1a3, Ndufa4i2, and Myl9 | Kcnk3 | 22 23 24 |
| Liver | PDGFRβ, NG2, CD146, α-SMA, RGS5, Desmin, PDGFRα, and Vimentin | 25 26 27 | |
| Heart | PDGFRβ, NG2, CD146, α-SMA, RGS5, Desmin, PDGFRα, Higd1b, SM-MHC, CD73, Nestin, Alp, Gli1, Notch3, Gm13889, and Rgs4 | Rgs4 | 22 28 29 |
| Bladder | PDGFRβ, NG2, CD146, α-SMA, RGS5, Pcp4l1, and Cox4i2 | Pcp4l1 | 22 24 30 31 32 |
| Kidney | PDGFRβ, NG2, CD146, α-SMA, RGS5, Desmin, Myh11, Kcna5, Cox4i2, Notch3, Gucy1a3, Ndufa4i2, Myl9, and Gm13889 | Myh11 and Kcna5 | 22 33 34 |
| Penis | PDGFRβ, NG2, CD146, α-SMA, RGS5, and Lbh | Lbh | 4 35 36 37 |
PDGFRβ: platelet-derived growth factor receptor beta; NG2: neural/glial antigen 2; CD146: melanoma cell adhesion molecule; α-SMA: alpha-smooth muscle actin; RGS5: regulator of G protein signaling 5; CD13: alanyl aminopeptidase, membrane; Ifitm1: interferon-induced transmembrane protein 1; Higd1b: HIG1 hypoxia inducible domain family member 1B; Kcnk3: potassium two-pore domain channel subfamily K member 3; Cox4i2: cytochrome c oxidase subunit 4I2; Gucy1a3: guanylate cyclase 1 soluble subunit alpha 1; Ndufa4i2: NDUFA4 mitochondrial complex associated like 2; Myl9: myosin light chain 9; PDGFRα: platelet-derived growth factor receptor alpha; SM-MHC: myosin heavy chain 11; CD73: 5’-nucleotidase ecto; Alp: alopecia, recessive; Gli1: glioma-associated oncogene homolog 1; Notch3: neurogenic locus notch homolog protein 3; Gm13889: chromosome 11 open reading frame 96; Rgs4: regulator of G protein signaling 4; Pcp4l1: Purkinje cell protein 4 like 1; Myh11: myosin heavy chain 11; Kcna5: potassium voltage-gated channel subfamily a member 5; Lbh: limb bud and heart development protein homolog
PENILE PERICYTE FUNCTION
Microvascular barrier function
Pericytes are recognized for their significant role in vascular development and the maintenance of BBB integrity.15 Pericytes do not induce BBB-specific gene expression in CNS endothelial cells; however, they suppress molecular expression that increases vascular permeability.41 Utilizing a dual-promoter strategy involving PDGFRβ and NG2, the loss of pericytes leads to a failure in the formation of tight junctions between endothelial cells, consequently resulting in abnormal BBB permeability.42 Given that the penis is a vascular organ with a specialized vascular bed, it is reasonable to speculate that pericytes also play an important role in maintaining the structural integrity of the blood vessels and regulating the permeability of penile tissues. Yin et al.4 demonstrated that enhancing pericyte function through the administration of hepatocyte growth factor (HGF) protein reduces corpus cavernous permeability and restores erectile function in diabetic mice.4 Subsequently, some related studies have demonstrated that pericytes can reduce the permeability of penile tissues through various signaling pathways. For example, pericyte-derived dickkopf2 restores endothelial cell junctions and enhances pericyte-endothelial cell interactions, thereby reducing cavernous vessel permeability.43 In addition, studies conducted by Anita et al.44 and Yin et al.45 revealed that pericyte-derived extracellular vesicle (EV)-mimicking nanovesicles promote neurovascular regeneration in mouse models of cavernous nerve injury, diabetic-induced ED, and sciatic nerve transection. Furthermore, studies conducted by Ock et al.46 and Yin47 also revealed that heme-binding protein 1 (HEBP1), delivered through pericyte-derived EVs, can regulate tight junctions (including claudin 1, claudin 2, claudin 3, and claudin 11), thereby modulating vascular permeability in mouse models of diabetes and neuropathic ED. In addition, pericytes have demonstrated protective effects against BBB disruption induced by hypoxia in vitro.48 Hypoxia represents a significant pathophysiological factor in ED,49 affecting various aspects, including nerves, blood vessels, endocrine function, and cytokines levels.49 For example, chronic hypoxia induces penile fibrosis and pro-fibrotic endothelin-1 receptor type B (ETB) overexpression, thereby reducing the contractile activity of endothelin-1 and nitric oxide formation.50 However, the precise mechanism underlying hypoxia-induced ED remains incompletely understood. Therefore, targeting penile pericytes presents a promising avenue to understand the specific mechanism of hypoxia-induced ED further.
Contractile function
Pericytes, similar to smooth muscle cells, express various contractile proteins such as: α-SMA, vimentin, tropomyosin, and myosin.51,52,53 Hibbs et al.54 shown that pericytes can control capillary diameter and regulate cerebral blood flow by responding to vasoactive stimuli through contraction and relaxation. Rucker et al.52 demonstrated that pericytes respond to vasoconstrictors such as angiotensin-II, serotonin, and vasodilators, including nitric oxide and cholinergic agonists, which was observed by measuring the surface area of collagen lattices in vitro. Pericytes adjust their contraction or relaxation based on their surrounding environment and exposure duration. Additionally, the signaling pathways regulating pericyte contraction or relaxation vary across different organs. For example, Speyer et al.55 demonstrated that lipopolysaccharide induces relaxation of lung pericytes through an inducible nitric oxide synthase-independent mechanism. In addition, Kerkar et al.56 demonstrated that reactive oxygen species metabolites (ROM) induce biphasic contractile responses in lung pericytes, depending on the duration of exposure to ROM. Furthermore, Chen et al.29 revealed that cardiac pericytes demonstrated similar myogenic capacity and contractile characteristics to cardiomyocytes. The mechanism underlying smooth muscle cell contraction during penile erection has been extensively studied. For example, the upregulation of α-SMA increases fibroblast contractile activity,57 while relaxation of arterial smooth muscle increases blood flow to the penis. Additionally, the contraction of trabecular smooth muscle leads to the opening of sinusoids in penile erectile tissue, a process mediated by two key proteins: myosin light chain kinase and myosin light chain phosphatase.58,59 Considering that pericytes express associated contractile proteins, it suggests that the contraction and relaxation of penile pericytes may also be significant in penile erection. Exploring the response mechanisms of penile pericytes contraction and relaxation holds promise for revealing valuable insights. This research may contribute significantly to the development of new therapeutic targets with substantial implications for the treatment of ED.
Immune regulation function
Pericytes have been shown to respond to various pro-inflammatory stimuli, leading to the expression of diverse pro-inflammatory cytokines through complex secretory responses.60 Many studies have shown that pericytes can regulate immune cell trafficking in multiple pathways. For example, pericytes play an important role in the migration of leukocytes across the endothelium into the interstitium.61 Additionally, pericytes promote neutrophil migration in an in vivo model of tumor necrosis factor-α (TNF-α)- or interleukin 1β (IL-1β)-stimulated mouse cremaster muscle.62,63 Furthermore, NG2+ pericytes guide interstitial leukocyte trafficking by upregulating the expression of intercellular adhesion molecule-1 and releasing the chemokine migration inhibitory factor.64 In addition, low-grade systemic inflammation is associated with ED development, which commonly coexists with conditions such as insulin resistance, obesity, type 2 diabetes, hypertension, and hyperlipidemia.65 Previous studies have shown elevated inflammatory biomarkers, such as interleukin 6, high-sensitivity C-reactive protein, IL-1β, and TNF-α, in both animal models and humans with ED.66,67,68 While Ruan et al.69 demonstrated that TNF-α could suppress endothelial nitric oxide synthase (eNOS) gene expression in endothelial cells, thereby causing endothelial damage and increasing the risk of ED, and Verma et al.70 have similarly shown that CRP can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, inducing an inflammatory endothelial phenotype by reducing the expression and activity of endothelial nitric oxide synthase, the precise mechanism remains poorly understood. Currently, no direct evidence supporting the significant role of penile pericytes in regulating ED-related inflammatory factors was observed. Further investigation into the immune aspects of penile pericytes may have important implications for the development of treatments targeting ED caused by various chronic inflammations.
Stem cell differentiation function
Pericytes possess stem cell potential.71 Influenced by the microenvironment, pericytes can differentiate into specific lineages, acquiring diverse morphological and functional properties such as those of smooth muscle cells, adipocytes, chondrocytes, osteocytes, fibroblasts, myocytes, immune cells, and neural cells.39,53 For example, under chronic inflammation conditions, pericytes have demonstrated the ability to differentiate into macrophages and dendritic cells, thereby mediating inflammation.72,73 Furthermore, bone marrow-derived pericytes progenitor cells have shown the capability to differentiate into mature pericytes, thereby regulating vessel stability and vascular survival.74 Pericytes within the human myocardium demonstrate angiogenic behavior under hypoxic conditions and show modest cardiogenic potential in vivo.29 In addition, Xu et al.75 demonstrated the potential use of pericytes in Duchenne muscular dystrophy treatment owing to their capacity for myogenic differentiation. Additionally, it is known that pericytes transplanted into severe combined immunodeficient mice can generate skeletal muscle fibers.76 While cell therapy has found applications in various therapeutic fields such as regenerative medicine, immune diseases, and cancer treatment,77 its utilization in addressing ED remains relatively nascent, as does stem cell therapy. Most cell-based therapies are still in the early stages of clinical development, primarily phase I and II trials.78,79 Therefore, a comprehensive investigation into the origin and differentiation pathways of pericytes may establish them as a promising source of therapeutic cells for many conditions, with particular potential in ED treatment.
PERICYTE IN DIFFERENT ED MODELS
Diabetes-associated ED model
Diabetic ED stands as a hallmark complication of diabetes, and diabetes is one of the most common causes of ED worldwide. Endothelial cells, smooth muscle cells, and pericytes are pivotal in the pathogenesis of diabetic ED. While the role of endothelial cells and smooth muscle cells in diabetic ED has been recognized for over 20 years, research on pericytes in this context is relatively recent, spanning less than10 years. Contrastingly, pericytes have been extensively studied in other diabetes-related complications, such as diabetic eye disease, diabetic nephropathy, and diabetic neuropathy.80,81,82 In these conditions, pericyte loss and dysfunction are implicated in vascular leakage, vascular apoptosis, hypoxia, and inflammation.82 Our analysis focused on pericyte research specific to diabetic ED within the past 10 years (Table 2).4,43,44,47,83,84,85,86,87,88,89,90,91 Most studies have shown that pericytes play a role in diabetic ED by reducing reactive oxygen species (ROS) production, permeability, and apoptosis and improving cell migration, cell survival, and vascular stability, thereby promoting penile angiogenesis (Figure 1 and 2). These processes involve the modulation of various signaling pathways regulating pericyte function. For example, platelet-derived growth factor B subunits (PDGF-BB) and PDGFRβ signaling are known to promote pericyte recruitment.92 Yin et al.4 provided initial evidence demonstrating that inhibition of PDGF-BB and PDGFRβ signaling, using an anti-PGDFRβ blocking antibody (APB5), significantly impaired erectile function both in vivo and in vitro. This suggests that pericytes play an important role in penile erection. Additionally, they demonstrated that HGF protein treatment increased cavernous pericyte content and restored erectile function in diabetic mice.4 Furthermore, Kwon et al.83 demonstrated that intracavernous injection of embryonic stem cell-derived extracellular vesicle-mimetic nanovesicles increased HGF expression, which ultimately induced pericyte proliferation and survival signaling. Subsequent experiments have consistently shown that most treatment options aimed at promoting pericyte function recovery and angiogenesis also stimulate the regeneration of blood vessels and nerves. They achieve this by activating cell survival pathways, decreasing cavernous permeability pathways, or inhibiting cell death pathways. Significantly, these treatments involve various signaling pathways, including the phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT)/eNOS/NF-κB p65, cell-to-cell junction, and pro-nerve growth factor (ProNGF) and p75 neurotrophin receptor (p75NTR).43,44,47,84,85,86,87,88,89,90,91 Despite these advancements, the specific roles and signaling pathways of pericytes in diabetic ED remain poorly understood. Significant amounts of basic research data are required to advance our understanding of the role of pericytes in diabetic ED. Utilizing single-cell analysis techniques could enable the examination of specific gene expressions and signaling pathways in pericytes under diabetic ED conditions. This approach promises to deepen our comprehension of pericyte involvement in diabetic ED and facilitate the development of increasingly effective treatment strategies for this condition.
Table 2.
Signaling pathways associated with pericytes in diabetic erectile dysfunction
| Signaling pathways: function | Methods | Species/tissue | Reference |
|---|---|---|---|
| PDGF-BB and PDGFRβ signaling: pericyte recruitment | Use APB5 antibody to blocking PDGF-BB and PDGFRβ signaling | Mouse/CC | 4 |
| HGF signaling: pericyte recruitment and coverage to endothelial cells and decrease cavernous permeability | Use recombinant HGF protein and ESC-NV to induce HGF signaling | Mouse/CC | 4 83 |
| ANG 1-Tie2 signaling: pericyte survival, decrease cavernous permeability and ROS production | Use Sac-1004 compound, DKK2 protein, soluble Tie2 antibody, HEBP1 protein, IGFBP5 short hairpin RNA to activate Tie2 signaling | Mouse/CC | 43 47 84 85 |
| ProNGF and p75NTR signaling: pericyte survival, increase cell-to-cell junction proteins and enhance neurite sprouting | Use proNGF antibodies or p75NTR small interfering RNA to activate ProNGF and p75NTR signaling | Mouse/CC | 86 |
| VEGF and angiopoietin signaling: pericyte survival, increase cell-to-cell junction proteins and angiogenic factors | Use DKK3 protein, peptides, or adenovirus and VASH1 protein to activate VEGF and angiopoietin signaling | Mouse/CC | 87 88 |
| LCN2-dependent activation of MAPK and PI3K/AKT, and suppress P53 signaling: peicyte survival and enhance neurite sprouting | Use PC-NV to activate MAPK and PI3K/AKT, and inhibit P53 signaling | Mouse/CC | 44 |
| TGF-β and BMP signaling: pericyte survival, enhance neurite sprouting and suppress fibrosis-related protein | Use BMP2 protein to activate BMP signaling | Mouse/CC | 89 |
| LRG1-LPHN2-induced PI3K, AKT, and NF-κB p65 signaling: pericyte survival, decrease cavernous permeability, and enhance neurite sprouting | Use LRG1 protein and LPHN2 short hairpin RNA to activate PI3K, AKT, and NF-κB p65 signaling | Mouse/CC | 90 |
| HSP70-CSE induced SDF1/HO-1/PI3K/AKT/eNOS/NF-κB p65 signaling: pericyte survival and enhance neurite sprouting | Use HSP70 protein to activate SDF1/HO-1/PI3K/AKT/eNOS/NF-κB p65 signaling | Mouse/CC | 91 |
PDGF-BB: platelet-derived growth factor-BB; PDGFRβ: platelet-derived growth factor receptor beta; CC: corpus cavernous; HGF: hepatocyte growth factor; ESC-NVs: embryonic stem cell-derived extracellular vesicle-mimetic nanovesicles; ANG1: angiopoietin 1; Tie2: tyrosine kinase with Ig and EGF homology domains 2; ROS: reactive oxygen species; DKK2: dickkopf-related protein 2; HEBP1: heme-binding protein 1; IGFBP5: insulin-like growth factor binding protein 5; NGF: nerve growth factor; NTR: neurotrophin receptor; VEGF: vascular endothelial growth factor; DKK3: dickkopf-related protein 3; VASH1: vasohibin 1; LCN2: lipocalin-2; MAPK: mitogen-activated protein kinase; PI3K: phosphoinositide 3-kinases; AKT: protein kinase B; PC-NVs: pericyte-derived extracellular vesicle-mimetic nanovesicles; TGF-β: transforming growth factor-β; BMP: bone morphogenetic protein; LRG1: leucine-rich α-2 glycoprotein 1; LPHN2: latrophilin 2; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; HSP70: 70-kDa heat shock proteins; CSE: cystathionine gamma-lyase; SDF1: stromal cell-derived factor-1 alpha protein; HO-1: heme oxygenase-1; eNOS: endothelial nitric oxide synthase
Figure 1.

The schema illustrates that pericytes restore diabetic ED or neurogenic ED by promoting the production of neurotrophic factors, reducing reactive oxygen species (ROS) production, reducing penile permeability and apoptosis, and improving cell migration, cell survival, and vascular stability. ED: erectile dysfunction; BDNF: brain-derived neurotrophic factor; NT-3: neurotrophin-3; NGF: nerve growth factor; PI3K: phosphoinositide 3-kinases; AKT: protein kinase B; JNK: Jun amino-terminal kinases; ERK: extracellular signal-regulated kinase; eNOS: endothelial nitric oxide synthase; Tie2: tyrosine kinase with Ig and EGF homology domains 2; Ox-LDL: oxidized low densitylipoprotein; ↑: upregulated; ↓: downregulated.
Figure 2.
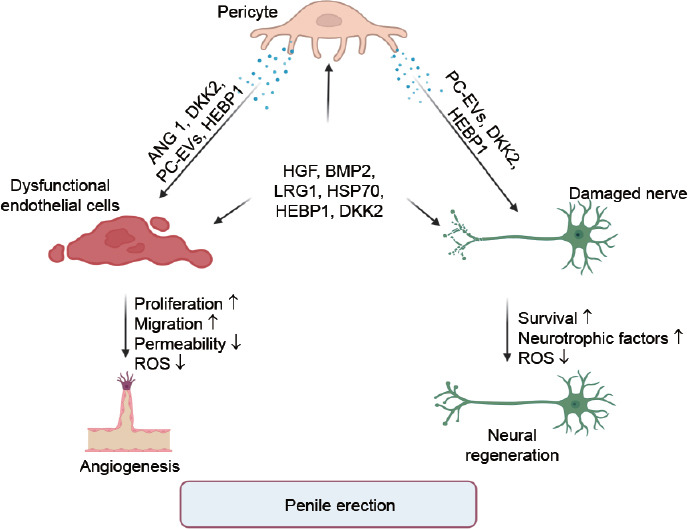
The diagram illustrates the role of recent targets in promoting angiogenesis and neural regeneration through pericytes. ANG1: angiopoietin 1; DKK2: dickkopf-related protein 2; PC-EVs: pericyte-derived extracellular vesicle; HEBP1: heme-binding protein 1; HGF: hepatocyte growth factor; BMP2: bone morphogenetic protein 2; LRG1: leucine-rich α-2 glycoprotein 1; HSP70: 70-kDa heat shock proteins; ROS: reactive oxygen species; ↑: upregulated; ↓: downregulated.
Nerve injury-induced ED model
Pericytes have been implicated in nerve regeneration, as they interact with nerve fibers to provide structural and molecular support for nerve growth and repair.93,94 A review study has focused on pericytes in the central nervous system, revealing their diverse functions, including angiogenesis, vasoconstriction, BBB maintenance, immune regulation, and modulation of glial scar formation.95 In addition, a recent study has demonstrated the role of peripheral nerve pericytes in forming and regulating the blood–nerve barrier (BNB).96 These pericytes influence BNB function and tight junction molecules through the secretion of various soluble factors, such as angiopoietin 1 (Ang1), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor.96 However, few articles have been published on the role of pericytes in neurogenic ED. In this review, we explore the potential pathological mechanisms of pericytes in ED induced by cavernous nerve injury (CNI). We found only five articles focusing on the pericytes in ED induced by CNI. Their mechanism is presented in Figure 1, 2 and Table 3.45,46,97,98,99 Ghatak et al.97 demonstrated that the wingless-related integration site (WNT) signaling-related dickkopf WNT signaling pathway inhibitor 2 (DKK2) protein might originate from pericytes. They found that DKK2 enhances nerve regeneration by secreting neurotrophic factors in a mouse model of cavernous nerve injury.97 In addition, Yin et al.100 demonstrated that pericyte-derived extracellular vesicle (EV)-mimetic nanovesicles (PC-NVs) promote nerve regeneration by increasing Schwann cell migration and neurite sprouting, and upregulating Akt, and eNOS-related cell survival signaling. Furthermore, findings from Ock et al.46 have indicated that Hebp1 delivered by mouse cavernous pericyte (MCP)-derived extracellular vesicles promotes neurovascular regeneration in CNI mice. This effect is achieved by reducing vascular permeability through the regulation of claudin family proteins and decreasing ROS production.46 Overall, these experiments collectively underscore the significant role of pericytes in neurogenic ED. Further research is imperative to understand the specific mechanisms by which pericytes contribute to neurogenic ED and other neurological diseases, leading to the identification of novel therapeutic targets and strategies.
Table 3.
Signaling pathways associate with pericytes in nerve injury-induced erectile dysfunction
| Signaling pathways: function | Methods | Species/tissue | Reference |
|---|---|---|---|
| WNT signaling: pericyte survival and enhance neurotrophic factors | Use DKK2 protein to activate WNT signaling | Mouse/CC | 97 |
| AKT/eNOS cell survival signaling: pericyte survival, enhance neurotrophic factors and neurite sprouting | Use PC-NV to activate AKT/eNOS cell survival signaling | Mouse/CC | 45 |
| ProNGF and p75NTR signaling: pericyte survival, enhance neurotrophic factors, cell-to-cell junction proteins, angiogenic factors, and neurite sprouting | Use proNGF antibodies and a proNGF antagonist small molecule (LM11A-31) to activate ProNGF and p75NTR signaling | Mouse/CC | 98 99 |
| HEBP1-induced tight junction signaling: pericyte survival, enhance neurite sprouting and decrease cavernous permeability or ROS production | Use HEBP1 protein to activate tight junction signaling | Mouse/CC | 46 |
WNT: wingless-related integration site; DKK2: dickkopf-related protein 2; CC: corpus cavernous; AKT: protein kinase B; eNOS: endothelial nitric oxide synthase; PC-NVs: pericyte-derived extracellular vesicle-mimetic nanovesicles; NGF: nerve growth factor; NTR: neurotrophin receptor; HEBP1: heme-binding protein 1; ROS: reactive oxygen species
CONCLUSIONS
Pericytes have been identified for over a hundred years; however, their role in various physiological and pathological conditions remains relatively understudied. As pivotal regulators within both the vascular and nervous systems, pericytes are involved in microvascular barrier function, contraction, immune response, stem cell differentiation, and particularly susceptible to dysfunction. When impaired, they can contribute to a range of vascular and neurological disorders. Recent studies have also shown that pericytes play an important role in the penile erection. This review delves into early findings on the role of pericytes in the penile erection, specifically in the diabetic ED and neurogenic ED. These studies found that restoring pericytes function reduced vascular and neuronal apoptosis, decreased cavernous permeability and ROS production, promote the secretion of neurotrophic factors, thereby restoring erectile function. Although some proteins and genes have been developed that can effectively restore pericytes function, the development and clinical availability of these proteins or genes require further validation. Therefore, there is a need to develop more and more effective therapeutic targets, especially to study the specific signaling pathways of pericytes in vascular regeneration and nerve regeneration, so as to determine new strategies for treating ED.
AUTHOR CONTRIBUTIONS
GNY and JKR conceived the study and wrote the draft. GNY drew the figures. JKR and GNY edited the review. Both authors read and approved the final manuscript.
COMPETING INTERESTS
Both authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by the Inha University Research Grant (to JKR). Figure 1 and 2 were created with BioRender.com (https://www.biorender.com/).
REFERENCES
- 1.Christ GJ. The penis as a vascular organ. The importance of corporal smooth muscle tone in the control of erection. Urol Clin North Am. 1995;22:727–45. [PubMed] [Google Scholar]
- 2.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379–95. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Han S, Su H, Li J, Zhi E, et al. Single-cell transcriptome atlas of the human corpus cavernosum. Nat Commun. 2022;13:4302. doi: 10.1038/s41467-022-31950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin GN, Das ND, Choi MJ, Song KM, Kwon MH, et al. The pericyte as a cellular regulator of penile erection and a novel therapeutic target for erectile dysfunction. Sci Rep. 2015;5:10891. doi: 10.1038/srep10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sims DE. The pericyte –a review. Tissue Cell. 1986;18:153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 8.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–8. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 9.Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther. 2017;171:30–42. doi: 10.1016/j.pharmthera.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales AL, Klug NR, Moshkforoush A, Lee JC, Lee FK, et al. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci U S A. 2020;117:27022–33. doi: 10.1073/pnas.1922755117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LS, Foster CG, Courtney JM, King NE, Howells DW, et al. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci. 2019;13:282. doi: 10.3389/fncel.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkler EA, Sagare AP, Zlokovic BV. The pericyte:a forgotten cell type with important implications for Alzheimer's disease?Brain Pathol. 2014;24:371–86. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao RN, Spurlock BO, Witherington R. Angiolymphoid hyperplasia with eosinophilia:report of a case with penile lesions. Cancer. 1981;47:944–9. doi: 10.1002/1097-0142(19810301)47:5<944::aid-cncr2820470521>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Yin GN, Ock J, Choi MJ, Song KM, Ghatak K, et al. A simple and nonenzymatic method to isolate human corpus cavernosum endothelial cells and pericytes for the study of erectile dysfunction. World J Mens Health. 2020;38:123–31. doi: 10.5534/wjmh.180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 16.Harari N, Sakhneny L, Khalifa-Malka L, Busch A, Hertel KJ, et al. Pancreatic pericytes originate from the embryonic pancreatic mesenchyme. Dev Biol. 2019;449:14–20. doi: 10.1016/j.ydbio.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–9. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, et al. Analysis of the brain mural cell transcriptome. Sci Rep. 2016;6:35108. doi: 10.1038/srep35108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia C, Keasey MP, Malone HM, Lovins C, Sante RR, et al. Vitronectin from brain pericytes promotes adult forebrain neurogenesis by stimulating CNTF. Exp Neurol. 2019;312:20–32. doi: 10.1016/j.expneurol.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth LC, Rustenhoven J, Scotter EL, Schweder P, Faull RL, et al. Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat. 2018;92:48–60. doi: 10.1016/j.jchemneu.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Mukouyama YS. Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med. 2018;5:78. doi: 10.3389/fcvm.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek SH, Maiorino E, Kim H, Glass K, Raby BA, et al. Single cell transcriptomic analysis reveals organ specific pericyte markers and identities. Front Cardiovasc Med. 2022;9:876591. doi: 10.3389/fcvm.2022.876591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts:busy multitaskers. Am J Pathol. 2016;186:2519–31. doi: 10.1016/j.ajpath.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashitani H, Mitsui R, Miwa-Nishimura K, Lam M. Role of capillary pericytes in the integration of spontaneous Ca2+ transients in the suburothelial microvasculature in situ of the mouse bladder. J Physiol. 2018;596:3531–52. doi: 10.1113/JP275845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva Meirelles L, Marson RF, Solari MI, Nardi NB. Are liver pericytes just precursors of myofibroblasts in hepatic diseases?Insights from the crosstalk between perivascular and inflammatory cells in liver injury and repair. Cells. 2020;9:188. doi: 10.3390/cells9010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostallari E, Shah VH. Pericytes in the liver. Adv Exp Med Biol. 2019;1122:153–67. doi: 10.1007/978-3-030-11093-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenoy PS, Bose B. Hepatic perivascular mesenchymal stem cells with myogenic properties. J Tissue Eng Regen Med. 2018;12:e1297–310. doi: 10.1002/term.2503. [DOI] [PubMed] [Google Scholar]
- 28.Alex L, Frangogiannis NG. Pericytes in the infarcted heart. Vasc Biol. 2019;1:H23–31. doi: 10.1530/VB-19-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WC, Baily JE, Corselli M, Diaz ME, Sun B, et al. Human myocardial pericytes:multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33:557–73. doi: 10.1002/stem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashitani H, Mitsui R, Shimizu Y, Higashi R, Nakamura K. Functional and morphological properties of pericytes in suburothelial venules of the mouse bladder. Br J Pharmacol. 2012;167:1723–36. doi: 10.1111/j.1476-5381.2012.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CY, Salazar MG, Miller S, Meyers C, Ding C, et al. Comparison of human tissue microarray to human pericyte transcriptome yields novel perivascular cell markers. Stem Cells Dev. 2019;28:1214–23. doi: 10.1089/scd.2019.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 33.Kramann R, Humphreys BD. Kidney pericytes:roles in regeneration and fibrosis. Semin Nephrol. 2014;34:374–83. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanska A, Kenyon C, Christian HC, Buckley C, Shaw I, et al. Human kidney pericytes produce renin. Kidney Int. 2016;90:1251–61. doi: 10.1016/j.kint.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Sun X, Bian J, Wu R, Guan X, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:e72790. doi: 10.1371/journal.pone.0072790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin GN, Wu J, Cui Y, Lin C, Shi L, et al. Transcriptional profiling of mouse cavernous pericytes under high-glucose conditions:implications for diabetic angiopathy. Investig Clin Urol. 2021;62:100–10. doi: 10.4111/icu.20200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae SG, Yin GN, Ock J, Suh JK, Ryu JK, et al. Single cell transcriptome analysis of cavernous tissues reveals the key roles of pericytes in diabetic erectile dysfunction. eLife. 2023;12:RP88942. doi: 10.7554/eLife.88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armulik A, Genove G, Betsholtz C. Pericytes:developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Cathery W, Faulkner A, Maselli D, Madeddu P. Concise review:the regenerative journey of pericytes toward clinical translation. Stem Cells. 2018;36:1295–310. doi: 10.1002/stem.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayloo S, Lazo CG, Sun S, Zhang W, Cui B, et al. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron. 2022;110:1641–55.e6. doi: 10.1016/j.neuron.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikolakopoulou AM, Montagne A, Kisler K, Dai Z, Wang Y, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089–98. doi: 10.1038/s41593-019-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin GN, Jin HR, Choi MJ, Limanjaya A, Ghatak K, et al. Pericyte-derived dickkopf2 regenerates damaged penile neurovasculature through an angiopoietin-1-Tie2 pathway. Diabetes. 2018;67:1149–61. doi: 10.2337/db17-0833. [DOI] [PubMed] [Google Scholar]
- 44.Anita L, Yin GN, Hong SS, Kang JH, Gho YS, et al. Pericyte-derived extracellular vesicle-mimetic nanovesicles ameliorate erectile dysfunction via lipocalin 2 in diabetic mice. Int J Biol Sci. 2022;18:3653–67. doi: 10.7150/ijbs.72243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin GN, Shin TY, Ock J, Choi MJ, Limanjaya A, et al. Pericyte-derived extracellular vesicles-mimetic nanovesicles improves peripheral nerve regeneration in mouse models of sciatic nerve transection. Int J Mol Med. 2022;49:18. doi: 10.3892/ijmm.2021.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ock J, Wu J, Liu FY, Fridayana FR, Niloofar L, et al. Heme-binding protein 1 delivered via pericyte-derived extracellular vesicles improves neurovascular regeneration in a mouse model of cavernous nerve injury. Int J Biol Sci. 2023;19:2663–77. doi: 10.7150/ijbs.81809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin GN. Pericyte-derived heme-binding protein 1 promotes angiogenesis and improves erectile function in diabetic mice. Investig Clin Urol. 2022;63:464–74. doi: 10.4111/icu.20220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, et al. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept. 2004;123:77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Niu LP, Zhu L, Adilijiang Y, Liu FX. [Mechanisms of hypoxia-induced erectile dysfunction:advances in studies. Zhonghua Nan Ke Xue. 2021;27:75–80. [Article in Chinese] [PubMed] [Google Scholar]
- 50.Vignozzi L, Morelli A, Filippi S, Vannelli GB, Mungai S, et al. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int J Impot Res. 2008;20:60–7. doi: 10.1038/sj.ijir.3901596. [DOI] [PubMed] [Google Scholar]
- 51.Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kilic K, et al. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. eLife. 2018;7:e34861. doi: 10.7554/eLife.34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–9. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZS, Zhou HN, He SS, Xue MY, Li T, et al. Research advances in pericyte function and their roles in diseases. Chin J Traumatol. 2020;23:89–95. doi: 10.1016/j.cjtee.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hibbs E, Love S, Miners JS. Pericyte contractile responses to endothelin-1 and abeta peptides:assessment by electrical impedance assay. Front Cell Neurosci. 2021;15:723953. doi: 10.3389/fncel.2021.723953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speyer CL, Steffes CP, Tyburski JG, Ram JL. Lipopolysaccharide induces relaxation in lung pericytes by an iNOS-independent mechanism. Am J Physiol Lung Cell Mol Physiol. 2000;278:L880–7. doi: 10.1152/ajplung.2000.278.5.L880. [DOI] [PubMed] [Google Scholar]
- 56.Kerkar S, Speyer C, Tyburski J, Steffes C. Reactive oxygen metabolites induce a biphasic contractile response in microvascular lung pericytes. J Trauma. 2001;51:440–5. doi: 10.1097/00005373-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–50. [PubMed] [Google Scholar]
- 59.Cripps SM, Mattiske DM, Pask AJ. Erectile dysfunction in men on the rise:is there a link with endocrine disrupting chemicals? Sex Dev. 2021;15:187–212. doi: 10.1159/000516600. [DOI] [PubMed] [Google Scholar]
- 60.Navarro R, Compte M, Alvarez-Vallina L, Sanz L. Immune regulation by pericytes:modulating innate and adaptive immunity. Front Immunol. 2016;7:480. doi: 10.3389/fimmu.2016.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14:232–46. doi: 10.1038/nri3641. [DOI] [PubMed] [Google Scholar]
- 62.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–34. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One. 2012;7:e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct'them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 65.Das UN. Is erectile dysfunction a low-grade systemic inflammatory condition? Eur Heart J. 2007;28:642–3. doi: 10.1093/eurheartj/ehl531. author reply 643–4. [DOI] [PubMed] [Google Scholar]
- 66.Billups KL, Kaiser DR, Kelly AS, Wetterling RA, Tsai MY, et al. Relation of C-reactive protein and other cardiovascular risk factors to penile vascular disease in men with erectile dysfunction. Int J Impot Res. 2003;15:231–6. doi: 10.1038/sj.ijir.3901012. [DOI] [PubMed] [Google Scholar]
- 67.Davies KP, Melman A. Markers of erectile dysfunction. Indian J Urol. 2008;24:320–8. doi: 10.4103/0970-1591.42612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamashita S, Kato R, Kobayashi K, Hisasue S, Arai Y, et al. Inhibition of interleukin-6 attenuates erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J Sex Med. 2011;8:1957–64. doi: 10.1111/j.1743-6109.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 69.Ruan Z, Xie X, Yu H, Liu R, Jing W, et al. Association between dietary inflammation and erectile dysfunction among US adults:a cross-sectional analysis of the National Health and Nutrition Examination Survey 2001-2004. Front Nutr. 2022;9:930272. doi: 10.3389/fnut.2022.930272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit?C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–50. [PubMed] [Google Scholar]
- 71.Ahmed TA, El-Badri N. Pericytes:the role of multipotent stem cells in vascular maintenance and regenerative medicine. Adv Exp Med Biol. 2018;1079:69–86. doi: 10.1007/5584_2017_138. [DOI] [PubMed] [Google Scholar]
- 72.Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52:127–42. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- 73.Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu J, Li D, Hsu CY, Tian Y, Zhang L, et al. Comparison of skeletal and soft tissue pericytes identifies CXCR4+ bone forming mural cells in human tissues. Bone Res. 2020;8:22. doi: 10.1038/s41413-020-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 77.El-Kadiry AE, Rafei M, Shammaa R. Cell therapy:types, regulation, and clinical benefits. Front Med (Lausanne) 2021;8:756029. doi: 10.3389/fmed.2021.756029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bisson I, Green E, Sharpe M, Herbert C, Hyllner J, et al. Landscape of current and emerging cell therapy clinical trials in the UK:current status, comparison to global trends and future perspectives. Regen Med. 2015;10:169–79. doi: 10.2217/rme.14.71. [DOI] [PubMed] [Google Scholar]
- 79.Chung DY, Ryu JK, Yin GN. Regenerative therapies as a potential treatment of erectile dysfunction. Investig Clin Urol. 2023;64:312–24. doi: 10.4111/icu.20230104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–88. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 81.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–12. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 82.Warmke N, Griffin KJ, Cubbon RM. Pericytes in diabetes-associated vascular disease. J Diabetes Complications. 2016;30:1643–50. doi: 10.1016/j.jdiacomp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Kwon MH, Song KM, Limanjaya A, Choi MJ, Ghatak K, et al. Embryonic stem cell-derived extracellular vesicle-mimetic nanovesicles rescue erectile function by enhancing penile neurovascular regeneration in the streptozotocin-induced diabetic mouse. Sci Rep. 2019;9:20072. doi: 10.1038/s41598-019-54431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Batbold D, Song KM, Park JM, Park SH, Lee T, et al. Sac-1004, a pseudo-sugar derivative of cholesterol, restores erectile function through reconstruction of nonleaky and functional cavernous angiogenesis in the streptozotocin induced diabetic mouse. J Urol. 2016;195:1936–46. doi: 10.1016/j.juro.2015.12.103. [DOI] [PubMed] [Google Scholar]
- 85.Ock J, Suh JK, Hong SS, Kang JH, Yin GN, et al. IGFBP5 antisense and short hairpin RNA (shRNA) constructs improve erectile function by inducing cavernosum angiogenesis in diabetic mice. Andrology. 2023;11:358–71. doi: 10.1111/andr.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen NM, Song KM, Choi MJ, Ghatak K, Kwon MH, et al. Inhibition of proNGF and p75(NTR) pathway restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. J Sex Med. 2019;16:351–64. doi: 10.1016/j.jsxm.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 87.Song KM, Kim WJ, Choi MJ, Kwon KD, Limanjaya A, et al. Vasohibin-1 rescues erectile function through up-regulation of angiogenic factors in the diabetic mice. Sci Rep. 2021;11:1114. doi: 10.1038/s41598-020-80925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song KM, Kim WJ, Choi MJ, Limanjaya A, Ghatak K, et al. Intracavernous delivery of Dickkopf3 gene or peptide rescues erectile function through enhanced cavernous angiogenesis in the diabetic mouse. Andrology. 2020;8:1387–97. doi: 10.1111/andr.12784. [DOI] [PubMed] [Google Scholar]
- 89.Kwon MH, Rho BY, Choi MJ, Limanjaya A, Ock J, et al. BMP2 restores erectile dysfunction through neurovascular regeneration and fibrosis reduction in diabetic mice. Andrology. 2024;12:447–58. doi: 10.1111/andr.13475. [DOI] [PubMed] [Google Scholar]
- 90.Yin GN, Kim DK, Kang JI, Im Y, Lee DS, et al. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function. Exp Mol Med. 2022;54:626–38. doi: 10.1038/s12276-022-00773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghatak K, Yin GN, Hong SS, Kang JH, Suh JK, et al. Heat shock protein 70 in penile neurovascular regeneration requires cystathionine gamma-lyase. World J Mens Health. 2022;40:580–99. doi: 10.5534/wjmh.210249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 93.Beazley-Long N, Durrant AM, Swift MN, Donaldson LF. The physiological functions of central nervous system pericytes and a potential role in pain. F1000Res. 2018;7:341. doi: 10.12688/f1000research.13548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laredo F, Plebanski J, Tedeschi A. Pericytes:problems and promises for CNS repair. Front Cell Neurosci. 2019;13:546. doi: 10.3389/fncel.2019.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng J, Korte N, Nortley R, Sethi H, Tang Y, et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018;136:507–23. doi: 10.1007/s00401-018-1893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimizu F, Sano Y, Abe MA, Maeda T, Ohtsuki S, et al. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol. 2011;226:255–66. doi: 10.1002/jcp.22337. [DOI] [PubMed] [Google Scholar]
- 97.Ghatak K, Yin GN, Choi MJ, Limanjaya A, Minh NN, et al. Dickkopf2 rescues erectile function by enhancing penile neurovascular regeneration in a mouse model of cavernous nerve injury. Sci Rep. 2017;7:17819. doi: 10.1038/s41598-017-17862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung DY, Song KM, Choi MJ, Limanjaya A, Ghatak K, et al. Neutralizing antibody to proNGF rescues erectile function by regulating the expression of neurotrophic and angiogenic factors in a mouse model of cavernous nerve injury. Andrology. 2021;9:329–41. doi: 10.1111/andr.12873. [DOI] [PubMed] [Google Scholar]
- 99.Yin GN, Ock J, Limanjaya A, Minh NN, Hong SS, et al. Oral administration of the p75 neurotrophin receptor modulator, LM11A-31, improves erectile function in a mouse model of cavernous nerve injury. J Sex Med. 2021;18:17–28. doi: 10.1016/j.jsxm.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 100.Yin GN, Park SH, Ock J, Choi MJ, Limanjaya A, et al. Pericyte-derived extracellular vesicle-mimetic nanovesicles restore erectile function by enhancing neurovascular regeneration in a mouse model of cavernous nerve injury. J Sex Med. 2020;17:2118–28. doi: 10.1016/j.jsxm.2020.07.083. [DOI] [PubMed] [Google Scholar]


