Abstract
Mouse spermatogenesis entails the maintenance and self-renewal of spermatogonial stem cells (SSCs), which require a complex web-like signaling network transduced by various cytokines. Although brain-derived neurotrophic factor (BDNF) is expressed in Sertoli cells in the testis, and its receptor tropomyosin receptor kinase B (TrkB) is expressed in the spermatogonial population containing SSCs, potential functions of BDNF for spermatogenesis have not been uncovered. Here, we generate BDNF conditional knockout mice and find that BDNF is dispensable for in vivo spermatogenesis and fertility. However, in vitro, we reveal that BDNF-deficient germline stem cells (GSCs) exhibit growth potential not only in the absence of glial cell line-derived neurotrophic factor (GDNF), a master regulator for GSC proliferation, but also in the absence of other factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and insulin. GSCs grown without these factors are prone to differentiation, yet they maintain expression of promyelocytic leukemia zinc finger (Plzf), an undifferentiated spermatogonial marker. Inhibition of phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), and Src pathways all interfere with the growth of BDNF-deficient GSCs. Thus, our findings suggest a role for BDNF in maintaining the undifferentiated state of spermatogonia, particularly in situations where there is a shortage of growth factors.
Keywords: BDNF, spermatogenesis, spermatogonia, stem cells
INTRODUCTION
Mammalian sperm production in seminiferous tubules depends on the self-renewal and differentiation of spermatogonial stem cells (SSCs).1 SSCs localize near the basement membrane of the seminiferous tubules and give rise to differentiating spermatogonia through the interaction with Sertoli cells, extracellular matrix, and/or interstitial blood vessels.2,3
If testicular cell suspension is placed in defined medium, only SSCs but not meiotic germ and somatic cells form colonies that can be stably cultured for a long term (called germline stem cells [GSCs]).4 GSCs exhibit remarkable characteristics. They can give rise to functional sperm once they are transplanted into the seminiferous tubules of competent mice.4 More recent studies established methods to grow GSCs under serum-free or feeder-free conditions, which have provided flexibility in the analyses of SSC growth and differentiation.4,5,6,7 Thus, one advantage of the cell line-based approach, including GSC experiments, is that a simplified model of complex interactions between molecules and cells in living organisms can be studied.
The standard GSC culture medium contains cytokines and peptide hormones such as glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and insulin.7,8 Among them, GDNF is especially important, as GDNF high-producing transgenic mice exhibited accumulation of undifferentiated spermatogonia in their testis, while the heterozygous GDNF knockout mice exhibited hypospermatogenesis and infertility.9,10 Furthermore, GSCs cannot be stably established in the absence of GDNF.11 GDNF transduces intracellular signals through specific receptors to the nucleus, which lead to the regulation of gene expression. Phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways are known to be mediated by GDNF,12,13 which could influence self-renewal and anti-apoptotic functions.
Brain-derived neurotrophic factor (BDNF) is another cytokine that affects germ cells and neurons by binding to the receptor tropomyosin receptor kinase B (TrkB).14,15 Studies in mice and humans have shown the important roles of BDNF both in the central and the peripheral nervous systems through TrkB, and BDNF mutations are associated with brain diseases such as Alzheimer’s disease, Huntington’s disease, depression, and schizophrenia.16 In the testis, BDNF is produced by Sertoli and Leydig cells, and TrkB is expressed in spermatogonia, thereby implicating BDNF-mediated interactions in germ cell development.17,18 However, the role and importance of BDNF on male germ cell development remain unclear.
Here, we generated mice and GSCs in which BDNF can be conditionally depleted by tamoxifen (Tmx). Our results show that BDNF knockout causes no observable adverse effect on mouse spermatogenesis. However, contrary to the normal GSCs, BDNF-depleted GSCs survive in the absence of an essential growth factor GDNF. Furthermore, we find that the BDNF-knockout GSCs are biased for a differentiating state in the absence of GDNF, EGF, bFGF, or insulin, suggesting a role for BDNF in maintaining the undifferentiated state in an unfavorable condition that lacks essential factors.
MATERIALS AND METHODS
Mice
The mice carrying floxed BDNF alleles (BDNFF/F); Rosa26-Cre recombinase-estrogen receptor T2 (CreERT2) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA), and they were crossed to obtain BDNFF/F; Rosa26-CreERT2 mice and BDNFF/+; Rosa26-CreERT2 mice. The mice were treated with 33 mg per kg body weight of Tmx (Sigma-Aldrich, St. Louis, MO, USA) for 5 days in 8 and 12 weeks of age by intraperitoneal injection to induce mutagenesis at the 2nd exon flanked by two locus of X-overP1 (loxP) sites of BDNF (Figure 1a). The testes were harvested at 16 weeks of age. The animal study was approved by the Committee for Animal Care and Use at Yokohama City University (Yokohama, Japan; Approval No. F-11-95 and No. F-A-14-013).
Figure 1.

BDNF is dispensable for mouse spermatogenesis in vivo. (a) Conditional knockout strategy for the BDNF gene by Tmx injection. Small horizontal arrowheads in the top scheme indicate genotyping primer positions for the detection of floxed (F) and CreERT2-recombined (FC) alleles. (b) Confirmation of knockout by genomic PCR using testicular DNA. The numbers indicate three independent animals. (c) Size of adult mouse testes comparing BDNFF/F and BDNFFC/FC (n = 3 mice per genotype) at 16 weeks of age. (d) Weight of adult testis (n = 3 mice per genotype; mice aged 16 weeks). Data are presented as mean±standard deviation of 6 testis samples. Two-sided Wilcoxon rank-sum test (P = 0.22). (e) IF images of seminiferous tubules (dotted lines) showing PLZF (green) and DAPI (white) from BDNFF/F and BDNFFC/FC testis. Yellow arrowheads: PLZF-expressing spermatogonia. Scale bars = 100 μm. (f) Representative IF images showing spermatogonia expressing PLZF (green) and/or KIT (magenta). Yellow arrowheads: PLZF+/KIT− spermatogonia; pink arrowheads: PLZF+/KIT+ spermatogonia; dotted lines: seminiferous tubules. Scale bars = 100 μm. (g) Box plots showing quantification of PLZF+/KIT− and PLZF+/KIT+ spermatogonia in BDNFF/F and BDNFFC/FC testis. n = 166 for BDNFF/F and n = 106 for BDNFFC/FC seminiferous tubules. NS: not significant; BDNF: brain-derived neurotrophic factor; PCR: polymerase chain reaction; PLZF: promyelocytic leukemia zinc finger; DAPI: 4’,6-diamidino-2-phenylindole; IF: immunofluorescence; CreERT2: Cre recombinase-estrogen receptor T2.
Genotyping polymerase chain reaction (PCR)
Genomic DNA extracted from mouse tail tips, testicular tissues, and GSCs was used to check knockout efficiency and confirm the Rosa26-CreERT2 allele using the following primers: BDNF, 5’-TGTGATTGTGTTTCTGGTGAC-3’ (forward), and 5’-TCAGGTCATGGATATGTCCAA-3’ (reverse); and Rosa26-CreERT2, 5’-GACCATGTCCAATTTACTGACCGTACAC-3’ (forward), and 5’-TTTTGCACGTTCACCGGCATCAACG-3’ (reverse). After PCR, F (approximately 1300 bp) and FC alleles (approximately 300 bp) of BDNF, as well as Rosa26-CreERT2 (400 bp), were identified by 2% agarose gel electrophoresis.
Immunofluorescence (IF)
Testes from BDNF mice were fixed and stained as described.19 Briefly, perfusion-fixation was performed with 2% paraformaldehyde (PFA) and testes were incubated in 2% PFA for 5 h at 4°C. After small incisions, tissues were mounted in Tissue-Tek O.C.T. compound (Sakura Finetek Japan, Tokyo, Japan). Sliced sections were treated with PBS containing 1% bovine serum albumin and incubated with a primary antibody for either 2 h at room temperature or overnight at 4°C. Samples were incubated with a secondary antibody for 1 h at room temperature. TSA Biotin System (Perkin Elmer, Boston, MA, USA) was used to amplify signals for the c-Kit proto-oncogene (KIT) staining, as reported previously.19 The following antibodies were used with indicated dilutions: anti-PLZF (1:200; AF2944; R&D systems, Minneapolis, MN, USA), anti-KIT (1:1500; ab112177; Abcam, Cambridge, MA, USA), Alexa 488 goat IgG (1:200; A11034; Life Technologies, Grand Island, NY, USA), and Biotin rat IgG (1:1000; 712-066-153; Jackson ImmunoResearch, West Grove, PA, USA). DNA was stained with 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Samples were mounted using ProLong Gold (Thermo Fisher, San Jose, CA, USA), and images were obtained using microscopy (FV-1000; Olympus, Tokyo, Japan).
GSC establishment
GSCs were established according to previous reports.4,5 Briefly, we used testes from BDNFF/F; Rosa26-CreERT2 mice and BDNFF/+; Rosa26-CreERT2 mice at postnatal day (P) 7–10. After the removal of the tunica albuginea, seminiferous tubules were dissociated using 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA) at 37°C for 15 min. The cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/10% fetal calf serum (FCS) and cultured on a gelatin-coated dish with GSC medium containing Stem Pro-34 (Thermo Fisher).4 The following were added to the medium; 15 ng ml−1 of recombinant human GDNF (450-10; PeproTech, Rocky Hill, NJ, USA), 20 ng ml−1 of recombinant murine EGF (315-09; PeproTech), 10 ng ml−1 of recombinant human bFGF (100-18B; PeproTech), and 25 μg ml−1 of recombinant human insulin (097-06474; Wako Chemicals, Osaka, Japan).
Reverse transcription-PCR (RT-PCR)/quantitative RT-PCR (qRT-PCR)
Total RNA extracted from 1×105 GSCs and mouse embryonic fibroblasts (MEFs) using Isogen (TOYOBO, Osaka, Japan) was treated with RQ1 DNase (Promega, Madison, WA, USA) and used to construct complementary DNA (cDNA) using either Superscript III (Thermo Fisher) or Superscript IV (Thermo Fisher). After 1:20-dilution of cDNA, target genes were amplified with 30 cycles of PCR for RT-PCR analysis by gel electrophoresis. The following primers were used for RT-PCR: BDNF, 5’-AGCTGAGCGTGTGTGACAGT-3’ (forward), and 5’-ACCCATGGGATTACACTTGG-3’ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (Gapdh), 5’-CAATGTGTCCGTCGTGGATCT-3’ (forward), and 5’-GCCTGCTTCACCACCTTCTT-3’ (reverse). For qRT-PCR, the DNase-treated cDNA (1:20-diluted) was amplified with FastStart Universal SYBR Green Master (Roche, Mannheim, Germany) using CFX96 (Bio-Rad, Redmond, WA, USA) to quantify messenger RNA (mRNA) expression. The following primers were used for qRT-PCR: Plzf, 5’-AACGGTTCCTGGACAGTTTG-3’ (forward), and 5’-CCATGTCCGTGCCAGTATG-3’ (reverse); Kit, 5’-CGCCTGCCGAAATGTATG-3’ (forward), and 5’-GGGTTGCAGTTTGCCAAG-3’ (reverse); stimulated by retinoic acid gene 8 (Stra8), 5’-ACCTGCAAGATGGGAATCCC-3’ (forward), and 5’-GGGACTGTCCTGAAGAAAACTG-3’ (reverse); and hypoxanthine phosphoribosyltransferase 1 (Hprt1), 5’-GCCCCAAAATGGTTAAGGTT-3’ (forward), and 5’-CAAGGGCATATCCAACAACA-3’ (reverse). For Gapdh expression, the primers indicated above were used. The expression levels were normalized for Hprt1 and Gapdh.
Establishment of BDNFF/F and WT MEFs
After removal of the viscera and head, either BDNFF/F or BDNF+/+ wild-type (WT) mouse embryos carrying Rosa26-CreERT2 at 13.5 days were washed with 5 mmol l−1 EDTA/phosphate-buffered saline (PBS) and minced with sterile scissors and stirred for 20 min at 37°C in water bath in the medium containing 0.1% trypsin/2 mmol l−1 EDTA and RQ1 DNase (1 unit ml−1). The suspension was further dissociated by pipetting and filtrated using a 100-μm strainer. The cells were cultured on gelatinized dishes in DMEM/10% FCS and passaged every 2–3 days with a dilution ratio of 1:5. The MEFs at passage 5 or 6 were used as the feeder layer for the GSCs.
GSC maintenance
GSCs were cultured at 37°C in 5% CO2 and 95% humidity. BDNFFC/FC cells were generated by the addition of 4-hydroxytamoxifen (4-OH-Tmx; Sigma-Aldrich) at 1 μmol l−1 to the medium. After 6 h of incubation, cells were washed twice with PBS to remove 4-OH-Tmx and the culture was continued without 4-OH-Tmx on BDNFFC/FC or WT MEF feeder cells. BDNFFC/FC MEFs were used for BDNFFC/FC GSCs, and WT MEFs were used for BDNFFC/+ GSCs unless otherwise indicated. For the time course analysis of the GSC growth, the cell number was counted with a hemocytometer at indicated timings.
Cytokine depletion and inhibitory experiments
For the depletion experiments, cytokines or insulin was removed from the culture medium, and the effects were examined after 8 days with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (M2128; Sigma-Aldrich). The qRT-PCR assay was done using the cells after 3 days of depletion to collect sufficient number of cells for RNA extraction. For the inhibitory experiments, each of the following inhibitors was added to each culture at the indicated concentration: PI3K inhibitor (LY294002), ERK inhibitor (PD0325901), and Src inhibitor (SU6656; Sigma-Aldrich). The viability was estimated by the MTT assay at the end of the culture.
MTT assay
Cellular viability was estimated by the MTT assay. For a 96-well plate, 500 μg ml−1 of MTT was added to the well and the plates were incubated at 37°C for 5 h in a CO2 incubator. Formazan formed in the medium was dissolved using acidified isopropanol for MTT. The absorbance at 595 nm and 730 nm (reference) was measured with a plate reader (Sunrise; Tecan, Hombrechtikon, Switzerland).
RESULTS
BDNF is dispensable for spermatogenesis
To explore the role of BDNF on spermatogenesis, we generated BDNF conditional knockout (BDNFFC/FC) mice by two rounds of Tmx injection to adult males. Our PCR using testicular tissues confirmed efficient deletion of 2nd exon flanked by two loxP sites of BDNF (Figure 1a and 1b). However, BDNF knockout males were healthy and fertile and there was no apparent difference in the phenotypes between BDNF+/+ (WT), BDNFFC/+, and BDNFFC/FC mice. Furthermore, there was no visible difference in the testes between BDNFF/F and BDNFFC/FC (Figure 1c), including the sizes and weights of the testes (Figure 1c and 1d). Our IF analysis of testicular sections for PLZF, a representative spermatogonial marker,20 suggested that BDNF control and knockout seminiferous tubules contained comparable amounts of undifferentiated spermatogonia as well as different types of germ cells (Figure 1e). To investigate spermatogonial maintenance and differentiation potential in detail, we performed staining for the KIT protein, which marks differentiation-committed progenitor spermatogonia and differentiating spermatogonia.20,21,22 Analysis of PLZF+/KIT− (undifferentiated spermatogonia) and PLZF+/KIT+ (progenitor spermatogonia) showed that the number of either type of spermatogonia in seminiferous tubules was not statistically different between BDNF control and knockout (Figure 1f and 1g). Thus, there was no apparent abnormality in the testicular histology and spermatogonial maintenance/differentiation in the knockout testis.
BDNF-depleted GSCs grow without GDNF
To investigate the possible functions of BDNF in spermatogonia more closely, we established GSCs from BDNFF/F; Rosa26-CreERT2 and BDNFF/+; Rosa26-CreERT2 mouse testes. GSCs have advantages compared to in vivo analyses as the effect of various physiological conditions in the knockout system can be readily examined. Notably, our RT-PCR analysis for the expression of BDNF in GSCs as well as the MEF feeder cells, which were used for the GSC culture, showed that BDNF is weakly expressed in GSCs and more strongly in MEFs (Figure 2a). This suggests a possible function of BDNF in spermatogonia. For the cultivation of the GSCs, we generated BDNFF/F; Rosa26-CreERT2 feeder cells, to exclude the possible influence of MEF-derived BDNF on the knockout GSCs. Using RT-PCR, we confirmed the loss of BDNF expression in the BDNFFC/FC MEFs after 4-OH-Tmx treatment (Figure 2b). We also confirmed efficient deletion of exon 2 in the knockout GSCs and MEFs using PCR after 4-OH-Tmx treatment (Figure 2c and 2d). Using these cells, we assessed the growth based on cell number counts with or without the addition of the essential cytokine GDNF to the medium. In the GDNF− medium, control GSC growth was almost completely abolished under the normal condition having BDNF (BDNFFC/+; Figure 2e). On the other hand, BDNF knockout GSCs showed a substantial proliferative/survival effect in the GDNF− medium (BDNFFC/FC; Figure 2f). The BDNF knockout GSCs grew less than the control cells in the GDNF+ medium, but there was little effect of GDNF withdrawal on the BDNF knockout GSCs after 27 days of culture (Figure 2e and 2f). These results suggest that the biological effect of BDNF on GSCs varies depending on the presence or absence of the GDNF signal.
Figure 2.

BDNFFC/FC GSCs grow without GDNF. (a) RT-PCR analysis for BDNF mRNA expression in WT MEFs and GSCs. Gapdh was used as the internal control. (b) RT-PCR confirmation for BDNF mRNA depletion in BDNFFC/FC MEFs. Gapdh was used as the internal control. (c) PCR confirmation of the conditional deletion of BDNF in GSCs after 4-OH-Tmx addition to media. Primers indicated in Figure 1a were used. (d) Knockout confirmation in MEFs by PCR. GSCs were cultured in GSC medium containing Stem Pro-34. Growth of (e) BDNFFC/+ and (f) BDNFFC/FC GSCs with or without GDNF. The growth rate was calculated by cell count at indicated timings. Data are presented as the mean±standard deviation of triplicate samples. Significant P values between GDNF+ and GDNF− are indicated (one-sided t-test). BDNF: brain-derived neurotrophic factor; PCR: polymerase chain reaction; RT-PCR: reverse transcription-PCR; mRNA: messenger RNA; GDNF: glial cell line-derived neurotrophic factor; Gapdh: glyceraldehyde-3-phosphate dehydrogenase; MEF: mouse embryonic fibroblast; GSC: germline stem cell; N: negative control; WT: wild-type; CreERT2: Cre recombinase-estrogen receptor T2.
BDNF knockout GSCs grow independent of essential cytokines
To investigate whether the loss of BDNF affects the dependency of GSCs on other cytokines, we then examined the effect of two additional factors, EGF and bFGF. We cultivated the BDNFFC/+ or BDNFFC/FC GSCs in medium containing different combinations of cytokines for 8 days and evaluated the cell viability by the MTT assay. In line with the cellular growth potential independent of GDNF (Figure 2f), the viability of BDNF knockout GSCs in GDNF− medium was higher than heterozygous GSCs, with 85.1% and 60.6% viability, respectively, as compared with the complete medium, although not statistically significant (P = 0.226; Figure 3a). Moreover, BDNF knockout GSC viability was not fully abolished in GDNF−/EGF−/bFGF− medium (52.1% viability as compared with the complete medium), which contrasted with the control GSCs (7.5%). Although there was a smaller effect of either EGF or bFGF depletion alone on the knockout GSCs, combined depletion of two or three factors showed a drastic difference between control and knockout GSCs, suggesting that BDNF-deficient GSCs depend less on all three cytokines. To test whether BDNF secreted from MEFs influences the dependency of knockout GSCs on cytokines, we performed a reciprocal experiment in which converse genotypes of MEFs were used. This experiment showed only a minimum or moderate viability difference on each factor between control and knockout GSCs (Figure 3b). Thus, supplementation of BDNF from MEFs likely rescues the BDNF knockout GSC phenotype.
Figure 3.
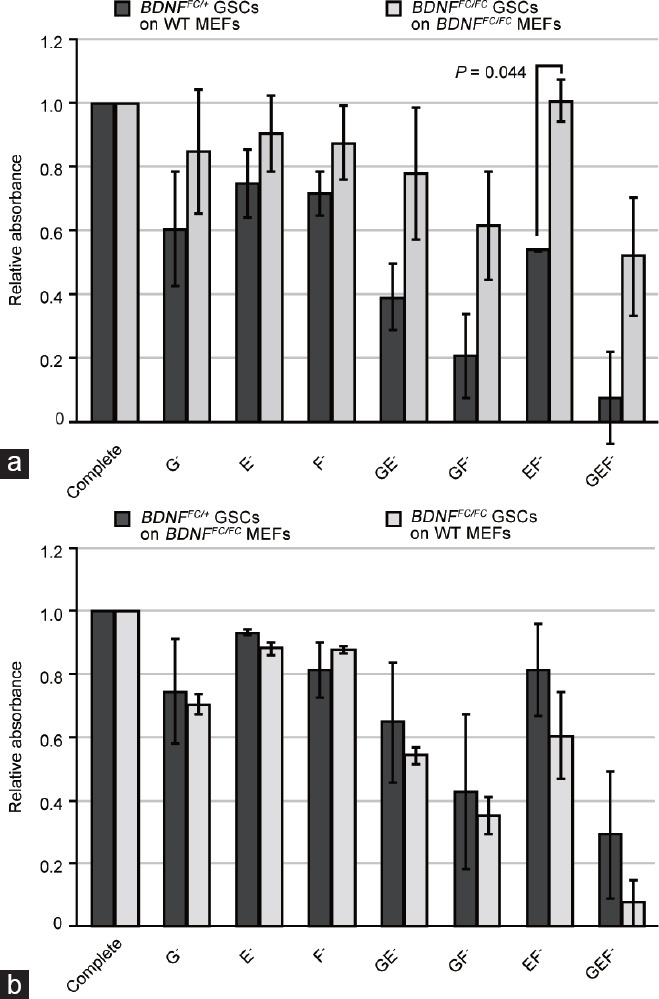
BDNF knockout GSCs grow without crucial cytokines. (a) Viability of BDNFFC/+ and BDNFFC/FC GSCs grown on corresponding genotypes of MEFs in the absence of GDNF (G), EGF (E), or bFGF (F). The complete medium contains all three factors. Cell viability was determined by the MTT assay after 8 days of culture. Mean absorbance values relative to the complete medium condition from duplicate samples are shown. (b) Control experiment showing viability of BDNFFC/+ GSC on BDNFFC/FC MEFs and BDNFFC/FC GSCs on WT MEFs from duplicate samples. Data are presented as mean±standard deviation. Significant P values are indicated (one-sided t-test). BDNF: brain-derived neurotrophic factor; MEF: mouse embryonic fibroblast; GSC: germline stem cell; WT: wild-type; GDNF: glial cell line-derived neurotrophic factor; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor.
Insulin signaling is crucial for spermatogenesis and GSC maintenance in mammals as well as in other animals.7,23,24,25 To evaluate the cellular response to insulin depletion, we cultured GSCs in the absence of insulin in the medium. This experiment showed a slightly decreased viability of both control and knockout GSCs in the insulin− medium, but higher viability of the knockout GSCs as compared with the heterozygous GSCs (Figure 4). Thus, these results suggest that the lack of BDNF cancels the essential requirement of GSCs for GDNF, EGF, bFGF, as well as insulin for their maintenance.
Figure 4.
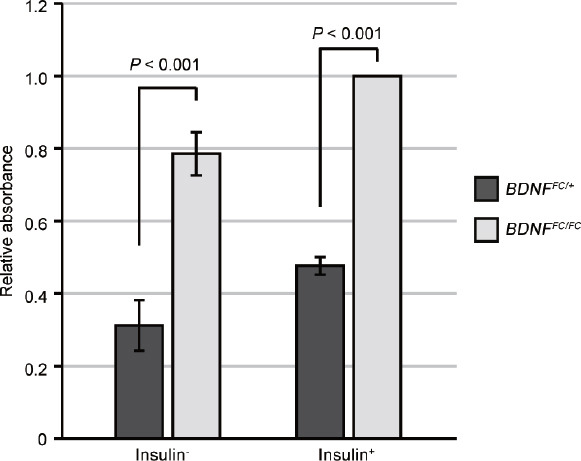
BDNF-depleted GSC growth without insulin. GSC growth with or without insulin in the absence of cytokines (GDNF, EGF, and bFGF). Data are presented as mean±standard deviation of duplicate samples. Significant P values are indicated (one-sided t-test). BDNF: brain-derived neurotrophic factor; GSC: germline stem cell; GDNF: glial cell line-derived neurotrophic factor; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor.
BDNF knockout GSC growth depends on PI3K and MAPK/ERK pathways
Studies suggest that the self-renewal of GSCs or SSCs depend on both PI3K and MAPK/ERK signaling pathways.26,27,28,29 The signals transduced from the binding of GDNF to the Ret/GDNF family receptor α 1 (GFRα1) receptor activate these pathways.27,30,31,32 Given that BDNF knockout GSCs can grow without GDNF, it is important to know whether these pathways remain active in the knockout GSCs in the GDNF− medium. To examine this, we evaluated the BDNF knockout GSC growth in the presence of varying concentrations of biochemical inhibitors targeted to the proteins in these pathways.
The addition of specific inhibitors against the PI3K signaling pathway, LY294002 (PI3K inhibitor), for the GSC culture reduced the viability of BDNF knockout GSCs irrespective of the addition of GDNF (Figure 5a). Likewise, culture with an inhibitor for mitogen-activated protein kinase 1 and 2 (MEK1/2), PD0325901 led to a decreased viability of BDNFFC/FC GSCs both in the presence and absence of GDNF (Figure 5b). Src functions as an upstream factor of the PI3K or MAPK/ERK pathways and is involved in GSC growth.33 GSC culture with Src family kinase inhibitors, SU6656, also confirmed the decreased viability of the BDNF knockout GSCs under increasing concentrations (Figure 5c). Together, these results suggest that PI3K and MAPK/ERK pathways, likely through the Src family kinases, are necessary for the GSC maintenance in the absence of BDNF irrespective of the addition of GDNF.
Figure 5.

Dependency of GSCs on the PI3K, MAPK/ERK, and Src pathways. (a) BDNFFC/FC GSC viability with the addition of PI3K inhibitor LY294002 for 8 days at the concentrations indicated. (b) BDNFFC/FC GSC viability in the presence of a MEK1/2 inhibitor PD0325901 for 8 days at the concentrations indicated. (c) Effect of another Src family kinase inhibitor SU6656 for 8 days on BDNFFC/FC GSC viability at the concentrations indicated. Data are presented as mean±standard deviation of duplicate samples. Significant P values are indicated (one-sided t-test). BDNF: brain-derived neurotrophic factor; GSC: germline stem cell; GDNF: glial cell line-derived neurotrophic factor; PI3K: phosphoinositide 3-kinase; MAPK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinase.
BDNF knockout GSCs are prone to differentiation in the absence of cytokines or insulin
The enhanced growth and viability of BDNF knockout GSCs without essential factors may be facilitated by changes in cellular fate. To evaluate this possibility, we performed qRT-PCR for Plzf, Kit, and Stra8 using the BDNF control and knockout GSCs. Consistent with the histological observations (Figure 1f and 1g), BDNF control and knockout GSCs grown in normal conditions containing essential factors expressed similar levels of an undifferentiated marker (Plzf) and differentiating markers (Kit and Stra8), as shown in Figure 6a. In the GDNF−, EGF−/bFGF−, and insulin− conditions, BDNF control and knockout GSCs showed similar expression levels of Plzf (Figure 6b–6d). In contrast, Kit and Stra8 mRNA levels increased significantly in the BDNF knockout GSCs in all three groups lacking GDNF, EGF/bFGF, or insulin (Figure 6b–6d). These results indicate that the BDNF knockout GSCs are prone to differentiation in the absence of essential factors when maintaining the characteristics of undifferentiated spermatogonial signature.
Figure 6.
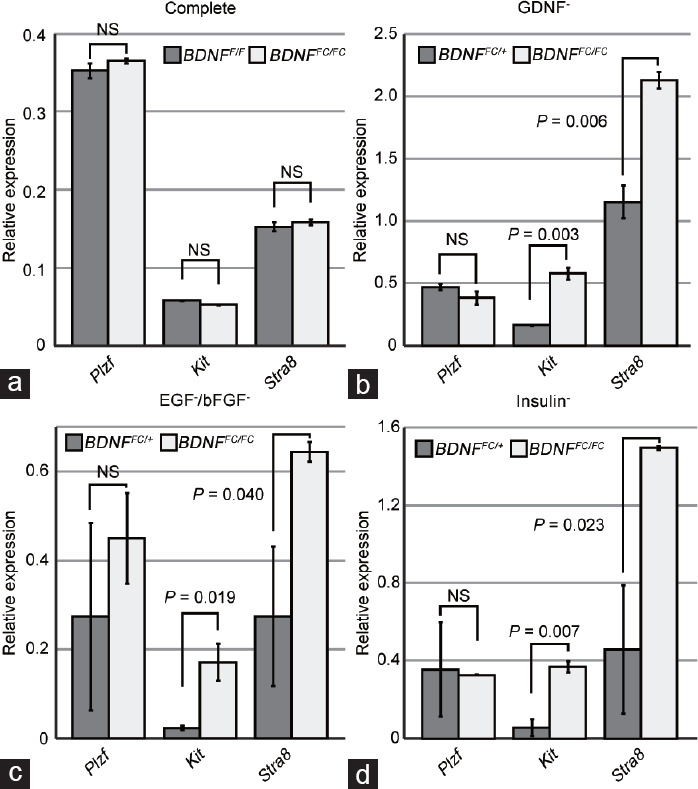
Spermatogonial marker mRNA expression in GSCs with or without essential factors. (a) qRT-PCR analysis of BDNFF/F and BDNFFC/FC GSCs cultured in the complete medium for the expression of spermatogonial markers. (b) qRT-PCR results showing gene expression in GDNF− medium. (c) qRT-PCR results showing gene expression in EGF−/bFGF− medium. (d) qRT-PCR results showing gene expression in insulin− medium. GSCs were cultured for 3 days after depletion of each factor. BDNF+/+ and BDNFFC/FC MEFs were used for control (BDNFF/F or BDNFFC/+) and knockout (BDNFFC/FC) GSCs, respectively. Data are presented as mean±standard deviation of duplicate samples normalized for the expression of Gapdh and Hprt1. Significant P values are indicated (one-sided t-test). NS: not significant; qRT-PCR: quantitative reverse transcription polymerase chain reaction; BDNF: brain-derived neurotrophic factor; MEF: mouse embryonic fibroblast; GSC: germline stem cell; mRNA: messenger RNA; GDNF: glial cell line-derived neurotrophic factor; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor; Gapdh: glyceraldehyde-3-phosphate dehydrogenase; Hprt1: hypoxanthine phosphoribosyltransferase 1.
DISCUSSION
BDNF is a neurotrophic factor that plays crucial roles in neuronal growth, survival, and development and is involved in various neuronal diseases in humans.16 Despite the reported expression of BDNF in the testis, the potential function in the germ cells has not been explored. In this study, using a conditional knockout system, we demonstrated that BDNF is dispensable for adult in vivo spermatogenesis. However, unlike normal GSCs, we found that BDNF-depleted GSCs survive and differentiate in the absence of a crucial self-renewal factor GDNF. BDNF-depleted GSCs also showed a trend of increased viability and differentiation in the conditions lacking EGF, bFGF, or insulin. This trend was observed more strongly with combinations of two or more growth factors. Thus, these results suggest the presence of a mechanism involving BDNF in maintaining the undifferentiated state of spermatogonia in the absence of growth factors, a mechanism not discernible through in vivo knockout mouse analysis.
In the testis, BDNF is primarily expressed from Sertoli and Leydig cells and likely transduces signals in SSCs through binding to TrkB.14,17 SSC homeostasis is also regulated by the cyclical expression of GDNF. GDNF is highly expressed in stages I to VII of the seminiferous epithelium cycle and controls stage-specific SSC self-renewal.34 Thus, SSCs may sense GDNF secretion levels and respond differently depending on the stage. Our data showed that GSCs grow and proliferate if the levels of both GDNF and BDNF are high. However, GSCs fail to survive in culture when GDNF alone is insufficient. In contrast, GSCs survive and start to differentiate if both BDNF and GDNF are absent. These GSCs showing expression of both undifferentiated and differentiating marker genes resemble transit-amplifying progenitor spermatogonia that express both PLZF and KIT20,21,22 or may share characteristics of a GDNF-independent GSC subpopulation.35 Thus, one role of BDNF could be to prevent the exhaustion of SSCs in the GDNF-low stages. Stage-independent expression of BDNF and TrkB is compatible with this hypothesis.17
The crosstalk between BDNF and GDNF and the combined effect of BDNF and GDNF have been demonstrated in neurons.36,37,38,39 Consistently, in GSCs, the presence of both BDNF and GDNF promoted growth most effectively. This situation may be similar to the crosstalk of other pathways, including the TGF-β and Notch signaling pathways, that can affect cellular growth in a context-dependent manner.40,41 Further studies are needed to clarify how BDNF takes effect in the lack of GDNF in GSCs.
Meanwhile, BDNF knockout GSC viability depended on PI3K, MAPK/ERK, and Src pathways. This observation suggests that the fundamental cascades required for GSC growth are somehow activated in the BDNF-depleted GSCs.27 Understanding the mechanisms involving BDNF and GDNF would lead to the development of defined culture methods of GSCs and provide insights into the studies of reproductive biology and male infertility.
AUTHOR CONTRIBUTIONS
ST and KK carried out the mouse, GSC, and molecular experiments and drafted the manuscript. MO and KN carried out the IF analysis. KO conceived the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We are grateful to Dr. Keiichiro Yoshida, Mr. Yasuyuki Sato, and Mr. Hidetoshi Sone (former members of Yokohama City University School of Medicine, Yokohama, Japan) for their valuable support and thank Dr. Rachel Fellows (Altos Labs, Cambridge, UK) for critically reading the manuscript. This work was partly supported by Grant-in-Aid for Scientific Research on Innovative Areas funding from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) KAKENHI (No. 22H04677 to KO), a grant from the Japan Society for the Promotion of Science KAKENHI (No. 20K09543 to ST, No. 20K07228 to KK, and No. 19KK0183 to KO), and the Takeda Science Foundation (2021 to KK).
REFERENCES
- 1.Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- 2.Russell LD. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. [Google Scholar]
- 3.O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. doi: 10.1016/j.semcdb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 5.Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, et al. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014;91:88. doi: 10.1095/biolreprod.114.122317. [DOI] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, et al. Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84:97–105. doi: 10.1095/biolreprod.110.086462. [DOI] [PubMed] [Google Scholar]
- 7.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 8.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–62. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 10.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 11.Kanatsu-Shinohara M. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development. 2005;132:4155–63. doi: 10.1242/dev.02004. [DOI] [PubMed] [Google Scholar]
- 12.De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, et al. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2000;60:3727–31. [PubMed] [Google Scholar]
- 13.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–73. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 14.Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 15.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor:implications in brain-related diseases. World J Biol Chem. 2014;5:409. doi: 10.4331/wjbc.v5.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Choi WS, Kwon H, Kwon YK. Temporal and spatial expression of neurotrophins and their receptors during male germ cell development. Mol Cells. 2001;12:360–7. [PubMed] [Google Scholar]
- 18.Müller D, Davidoff MS, Bargheer O, Paust HJ, Pusch W, et al. The expression of neurotrophins and their receptors in the prenatal and adult human testis:evidence for functions in Leydig cells. Histochem Cell Biol. 2006;126:199–211. doi: 10.1007/s00418-006-0155-8. [DOI] [PubMed] [Google Scholar]
- 19.Shirakawa T, Yaman-Deveci R, Tomizawa S, Kamizato Y, Nakajima K, et al. An epigenetic switch is crucial for spermatogonia to exit the undifferentiated state toward a kit-positive identity. Development. 2013;140:3565–76. doi: 10.1242/dev.094045. [DOI] [PubMed] [Google Scholar]
- 20.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–78. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates:are there differences from those in rodents? Reproduction. 2010;139:479–93. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, et al. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–16. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLean JA, Hu Z, Welborn JP, Song HW, Rao MK, et al. The RHOX homeodomain proteins regulate the expression of insulin and other metabolic regulators in the testis. J Biol Chem. 2013;288:34809–25. doi: 10.1074/jbc.M113.486340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–3. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 25.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–5. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–9. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa K, Namekawa SH, Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells. 2013;31:2517–27. doi: 10.1002/stem.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, et al. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Puri P, Phillips BT, Suzuki H, Orwig KE, Rajkovic A, et al. The transition from stem cell to progenitor spermatogonia and male fertility requires the SHP2 protein tyrosine phosphatase. Stem Cells. 2014;32:741–53. doi: 10.1002/stem.1572. [DOI] [PubMed] [Google Scholar]
- 30.He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, et al. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–78. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734–43. doi: 10.1242/dev.076539. [DOI] [PubMed] [Google Scholar]
- 32.Lucas BE, Fields C, Joshi N, Hofmann MC. Mono-(2-ethylhexyl)-phthalate (MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology. 2012;299:10–9. doi: 10.1016/j.tox.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on src family kinase signaling. J Biol Chem. 2007;282:25842–51. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Braun RE. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Development. 2018;145:dev151555. doi: 10.1242/dev.151555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takashima S, Kanatsu-Shinohara M, Tanaka T, Morimoto H, Inoue K, et al. Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports. 2015;4:1–14. doi: 10.1016/j.stemcr.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma HS. Post-Traumatic Application of Brain-Derived Neurotrophic Factor and Glia-Derived Neurotrophic Factor on the Rat Spinal Cord Enhances Neuroprotection and Improves Motor Function. Vienna: Springer; 2006. pp. 329–34. [DOI] [PubMed] [Google Scholar]
- 37.Zurn AD, Winkel L, Menoud A, Djabali K, Aebischer P. Combined effects of GDNF, BDNF, and CNTF on motoneuron differentiation in vitro. J Neurosci Res. 1996;44:133–41. doi: 10.1002/(SICI)1097-4547(19960415)44:2<133::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Ferrini F, Salio C, Boggio EM, Merighi A. Interplay of BDNF and GDNF in the mature spinal somatosensory system and its potential therapeutic relevance. Curr Neuropharmacol. 2021;19:1225–45. doi: 10.2174/1570159X18666201116143422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giehl KM, Schütte A, Mestres P, Yan Q. The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism. J Neurosci. 1998;18:7351–60. doi: 10.1523/JNEUROSCI.18-18-07351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morikawa M, Derynck R, Miyazono K. TGF-βand the TGF-βfamily:context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8:a021873. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopan R, Ilagan MX. The canonical Notch signaling pathway:unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


